Category:FL
Flavonoid (フラボノイド)
| Flavonoid Top | Molecule Index | Author Index | Journals | Structure Search | Food | New Input |
Contents |
Class Overview
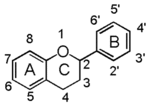
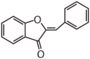
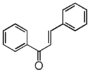
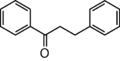
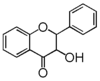
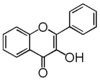
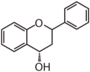
The word "flavonoid" comes from its Latin origin flavus (yellow) with oid, meaning yellow-ish. It comes from its history as yellow natural dye (quercetin and kaempferol are the most widespread flavone dyes. See flavone). Chemically speaking, it is a class of plant secondary metabolites that have two benzene rings (each called A-ring and B-ring) connected by a chain of three carbons (Figure 1).
The carbon chain, corresponding to the numbers 2,3,4 in Figure 1, is linked to a hydroxyl group in the A-ring to form the C-ring. The class of flavonoids are usually determined by the modification pattern of the C-ring (Table 1).
Flavonoid is utilized in many industrial processes from pigments to food additives. Often heard names include anthocyanin, catechin, flavan, and isoflavone. (BY Masanori Arita)
Biosynthesis 生合成
| Explanation (解説) | |
|---|---|
| Flavonoid is synthesized through the phenylpropanoid-acetate pathway in all higher plants (but not algae). Most plants contain the six major subgroups (chalcones, flavones, flavonols, flavandiols, anthycyanins, and proanthocyanidins), but the seventh, aurones, is not ubiquitous.
|
| 4-coumaroyl CoA + malonyl CoA | |||||||||||||||
| | |||||||||||||||
| CHALCONES, AURONES | chalcone | FLAVONES | |||||||||||||
| |
| ||||||||||||||
IFS |
FLAVANONE naringenin |
kaempferol | FLAVONOLS | quercetin | myricetin | ||||||||||
| ISO-FLAVONES | |
||||||||||||||
| DIHYDRO FLAVONOLS | dihydro-kaempferol | F3'H |
dihydro-quercetin | F3'5'H |
dihydro-myricetin | ||||||||||
| |
|
|
|||||||||||||
| LEUCOANTHO-CYANIDINS (flavan diols) |
leuco-pelargonidin | leuco-cyanidin | leuco-delphinidin | ||||||||||||
| |
PROANTHO- CYANIDINS | |
PROANTHO- CYANIDINS | |
PROANTHO- CYANIDINS | ||||||||||
| ANTHO-CYANIDINS | pelargonidin | |
cyanidin | |
delphinidin | | |||||||||
| |
epi-afzelechin | |
epi-catechin | |
epigallo-catechin | FLAVAN 3-OLS | |||||||||
| ANTHO-CYANINS | pelargonidin 3-glucoside | cyanidin 3-glucoside | delphinidin 3-glucoside | ||||||||||||
|
|
| |||||||||||||
| Information for the Gene Abbreviations (遺伝子略称の詳細) | |||
|---|---|---|---|
| Six Structural Genes (構造遺伝子) | |||
| Abbrev. | Name | Origin | Information |
| CHS | chalcone synthase | Bacterial polyketide synthases, particularly those in fatty acid synthesis (Verwoert et al. 1992) | Early response against light [1] [2] |
| CHI | chalcone-flavanone isomerase | Unclear and unique to plants.[3]
Eubacterium ramulus has the CHI activity. [4] |
Early response against light. |
| F3H | flavanone 3-hydroxylase | 2-oxoglutarate-dependent dioxygenase family [5] | Early response in Arabidopsis but late in Antirrhinum [6] |
| FLS | flavonol synthase | 2-oxoglutarate-dependent dioxygenase family [7] | Early response against light. In Arabidopsis, all structural genes are single-copy except for this one, to which six genes exist and two of them are not expressed. |
| DFR | dihydroflavonol 4-reductase | NADPH-dependent reductase associated with steroid metabolism [8] | Later response |
| ANS/LDOX | anthocyanidin synthase or leucoanthocyanidin dioxygenase | 2-oxoglutarate-dependent dioxygenase family | Later response |
| Auxiliary Genes (その他の遺伝子) | |||
| F3'H | flavonoid 3'-hydroxylase | cytochrome P450 hydroxylase family [9] | |
| F3'5'H | flavonoid 3',5'-hydroxylase | cytochrome P450 hydroxylase family [10] | Not reported in mosses or liverworts. The transformation of the F3'5'H and the cytochrome b5 gene of petunia into carnation changed its flower color deep purple.[11][12] |
| FSI | flavone synthase | Dioxygenase in parsley (FSI) and P450 monooxygenase in snapdragon (FSII). | |
| LAR (or LCR) | leucoanthocyanidin reductase | ||
| UF3GT | UDP flavonoid glucosyltransferase | ||
| GST | glutathione-S-transferase | Transport of flavonoids from cytoplasm to vacuole or cell walls requires both GST and the glutathione pump in ATP-binding cassette family.[13][14] | |
| AOMT | anthocyanin O-methyl transferase | ||
| |||
Bioactivity
| all flavonoids
photoprotectant, anti-oxidant |
全フラボノイド
抗紫外線、抗酸化作用 |
| Tannins (proanthocyanidins)
anti-bacteria, anti-fungi |
タンニン (プロアントシアニジニン)
抗菌、抗カビ作用 |
- ↑ Ryan KG, Swinny EE, Markham KR, Winefield C: Flavonoid gene expression and UV photoprotection in transgenic and mutant Penunia leaves. Phytochem 2002 59:23-32
- ↑ Li J, Ou-Lee T-M, Raba R, Amundson RG, Last RL: Arabidopsis flavonoid mutants are hypersensitive to UV-B irradiation. Plant Cell 1993 5: 171-179
Bioavailability
- Isoflavones
Isoflavones are efficiently absorbed from the colon and exhibit the highest bioavailability. (Usually polyphenols absorbed from the colon show very low availability.) Daidzein and genistein are known to form chlorinated products (e.g. 3- and 8-chlorodaidzein), then are conjugated with glucuronides and excreted in bile.
- Anthocyanins
Although anthocyanins comprise ca. 50% of total polyphenols, they are poorly available (less than 1% of intake in urinary levels). Anthocyanins are absorbed from the stomach, and their glycosides appear in plasma soon after the intake. In blood, only anthocyanins exist in a non-conjugated form.
- Flavan
Flavan 3-ols and phenolic acids are efficiently abosrbed from the small instestine, a few hours after intake.
- Others
Non-absorbed flavonoids are transported to the colon, and subjected to metabolism by microbiota. These flavonoids are therefore absorbed much less compared to flavan 3-ols and phenolic acids. Esterification of phenolic acids (e.g. chlorogenic acid), however, reduces absorption.
During absorption, polyphenols are metabolized to form β-glucuronide and sulfate conjugates (phase II conjugation in the intestinal wall), and catechol units are methylated. C-glycosides such as puerarin remain stable and not conjugated. These metabolized forms show markedly different bioactivities from their aglycones.
Links to familiar names 耳にする名前
- isoflavonoid in beans (豆のイソフラボン)
- anthocyanin in berries (ベリーのアントシアニン)
- catechin in tea (お茶のカテキン)
- rutin in buckwheat (ソバのルチン)
- hesperidin in orange (ミカンのヘスペリジン)
- naringenin chalcone in tomato (トマトのナリンゲニンカルコン)
Database statistics/ranking データベース統計
This database collects original references that report identification of flavonoid in various plant species. The database consists of three major namespaces: (flavonoid) compounds, plant species, and references. Currently, 6961 flavonoid structures, 3961 plant species, and 5215 references describing total 19861 metabolite-species relationships are registered.
Flavonoid content in food 食品中の量
| Category | Flavonol | Flavone | Flavan | Flavanone |
|---|---|---|---|---|
| Names | quercetin, kampferol, myricetin, isorhamnetin | apigenin, luteolin | catechin, epicatechin | |
| broccoli ブロッコリ | Δ | |||
| celery セロリ | ΔΔ | |||
| fava そら豆 | ΔΔ | |||
| hot pepper とうがらし | ΔΔ | |||
| onion たまねぎ | ΔΔ (Δ) | |||
| parsley パセリ | ΔΔΔ | |||
| peppermint ペパーミント | ΔΔ | |||
| spinach ほうれん草 | Δ | |||
| thyme タイム | ΔΔΔ | |||
| watercress クレソン | Δ | |||
| dill, fennel ディル, フェンネル | ΔΔΔ |
Δ 5 to <10 mg/100 g; ΔΔ 10 to <50 mg/100 g; ΔΔΔ 50< mg/100 g
The following vegetables and herbs have flavonoid contents less than 5 mg/100 g: beets, kidney beans, snap beans, cabbage, carrot, cauliflower, cucumber, endive, gourd, leek, lettuce, green peas, sweet pepper, potato, radish, tomato, oregano, perrilla, rosemary
Design of Flavonoid ID numbers ID番号の設計
12-DIGIT
| F | L | x | x | y | y | z | z | w | c | c | c |
- x ... backbone structure (母核構造)
FL1 aurone and chalcone; FL2 flavanone; FL3 flavone; FL4 Dihydroflavonol; FL5 Flavonol; FL6 Flavan; FL7 Anthocyanin; FLI Isoflavonoid; FLN Neoflavonoid
- y ... hydroxylation pattern in A and B ring (水酸基パターン)
Click above categories to see details. General description is here.
- z ... glycosylation pattern (糖修飾パターン)
Click above categories to see details. General description is here.
- w ... halogenation etc. (ハロゲン等)
Currently unused.
- c ... serial number (通し番号)
For Users of Flavonoid Viewer
The flavonoid IDs used in this site is the same as those in Flavonoid Viewer in metabolome.jp except for the following FL7 category.
| Anthocyanin glycosylated with other than glucose and galactose | ||
|---|---|---|
| Flavonoid Viewer FL7A..GS |
→ | This site FL7A..GO |
Subcategories
This category has the following 10 subcategories, out of 10 total.