Category:FL7
Anthocyanidin, Anthocyanin
| トップ | 化合物検索 | 著者索引 | 雑誌索引 | 構造検索 | 食品情報 | 新規入力 |
Upper classes : FL Flavonoid
| 2nd Class | |||
|---|---|---|---|
| FL7A | Anthocyani(di)n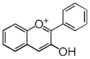
|
FL7D | 3-Deoxyanthocyani(di)n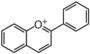
|
Contents |
アントシアニ(ジ)ンの概要
分布
|
Anthocyanin is almost ubiquitous, whereas 3-Deoxyanthocyanin (luteolinidin and apigeninidin) is found only in some lower plants (moss and fern[1][2][3]) and in sorghum (Sorghum bicolor [4]), gloxinia (Sinningia cardinalis) and many species in Gesneriaceae[5]. |
アントシアニンはほぼ植物全般に見つかりますが、3-デオキシアントシアニン(luteolinidin and apigeninidin)は下等植物(コケ、シダ)の他にはソルガム (Sorghum bicolor), グロキシニア (Sinningia cardinalis)およびイワタバコ科に属する種で見つかっています。 |
語源
|
The Greek word origin of anthocyanin is "flower" ἀνθός (anthos) and "blue" κυανός (kyanos). It was first used by Marquart to describe water-soluble pigments in red, blue, and purple from flowers [7]. Nowadays, such water-soluble pigments from flowers and fruits are called anthocyan. The flavonoid backbone without sugar (shown below) is called anthocyanidin (it is called aglycon because it is devoid of glyco moiety), and structure with sugars is called anthocyanin. Except for carotenoid (yellow) or indigo (deep blue), most pigments of plant origin are anthocyanins. |
アントシアニンの語源はギリシャ語で「花」意味するἀνθός (anthos)と, 「青」を意味するκυανός (kyanos)です。 Marquartにより花の赤、紫、青に対応する水溶性色素の意味で使われ始めました。今日、そうした水溶性色素はアントシアンと呼ばれます。そのうち、糖を持たず右上に示されたフラボノイド骨格だけの場合をアントシアニディンanthocyanidin (糖が無い形なのでa + glyco アグリコンと呼ばれます), 配糖体のものをアントシアニンanthocyaninと呼びます。 黄色のカロテノイドや藍色のインディゴを除くと、ほとんどの植物由来の色素はアントシアニンです。 |
- ↑ Bendz G, Martensson O, Terenlus L: Moss pigments I. The anthocyanins of Bryum cryophilum O. Mart. Acta Chem Scand 1962 16:1183-1190
- ↑ Bendz G, Martensson O: Moss pigments II. The anthocyanins of Bryum rutilans Brid. and Bryum weigelii Spreng. Acta Chem Scand 1963 17:266
- ↑ Comparative biochemistry of the flavonoids-II. 3-Desoxyanthocyanins and their systematic distribution in ferns and gesnerads. Phytochemistry 1966 5:589-600
- ↑ Nip WK, Burns EE: Pigment characterization in grain Sorghum. Cereal Chem 1969 46:490-495 also in 1971 48:74-80
- ↑ Harborne JB: Comparative biochemistry of the flavonoids-II. 3-Desoxyanthocyanins and their systematic distribution in ferns and gesnerads. Phytochemistry 5:589-600
- ↑ Piattelli M, Minale L: Pigments of centrospermae-II. Distribution of betacyanins. Phytochemistry 1964 3:547-557
- ↑ Marquart LC. "Die Farben der Bluethen. Eine chemisch-physiol." Abhandlung, Bonn, 1835 (Cited by Onslow MW. "The Anthocyanin Pigments of Plants," Cambridge University Press, 1925).
機能
花や野菜の色
|
Anthocyanin contains 3 aromatic rings, and glycosylation at the 3-OH position is necessary for stabilizing the aromatic ring. The general color of anchocyanin is red in acidic environment and purple/blue in alkali. The colors are, however, dependent on many other factors such as additional modifications and metal ions as is suggested by multi-color flowers such as pansy (yellow, orange, purple, violet, deep blue). (BY Masanori Arita) |
アントシアニンは芳香環を3つ有し, 3-OH位置への糖転移は芳香環を安定させるために必要といわれています。 色は一般的には酸性で赤、アルカリ性で青ですが、パンジーに様々な色(黄, オレンジ, 紫, 青, 濃紺など)があるように, 金属イオンや他の修飾基など様々な要素に左右されます。[1][2][3] |
防御物質
|
In sorghum, the 3-deoxyanthocyanidins are a class of phytoalexins, the class of compound to fend off fungi [4][5] They are produced within a cell under appressorial attack, first inside endoplasmic reticulum and then as colorless inclusions to the penetration site. The inclusions become yellow, and deep red with time. When the inclusions are broken by appressoria, the deoxyanthocyanidins kill the host cell and the pathogen together. |
ソルガムでは、デオキシアントシアニジンが、菌類から身を守るためのファイトアレキシン(防御物質)として作用します。菌類の付着器がついた細胞の小胞体でまず生成され、色のない小胞として感染部に移動します。小胞は次第に黄色、最後には濃赤に変化します。付着器の進入によってこの小胞が壊れると、デオキシアントシアニジンによって細胞は病原体もろとも死んでしまいます。 |
- ↑ Kroon J. et al. Plant J. 1994 Jan;5(1):69-80 PMID 8130799
- ↑ Boss PK. et al. Plant Mol Biol. 1996 Nov;32(3):565-9 PMID 8980508
- ↑ Fukuchi-Mizutani M. et al. Plant Physiol. 2003 Jul;132(3):1652-63 PMID 12857844 (research paper on Blue Rose)
- ↑ Snyder BA, Nicholson RL (1990) "Synthesis of phytoalexins in sorhum as a site-specific response to fungal ingress" Science 248:1637-1639 PMID 17746504
- ↑ Nicholson RL, Wood KV (2001) "Phytoalexins and secondary products, where are they and how can we measure them?" Physiol Mol Plant Pathol 59:63-69
生合成
|
The six common anthocyanidins are the product of three different branches. In the figure below, horizontal shifts are hydroxylation and vertical, methylation. |
6種の代表的アントシアニジンは、3つの合成経路に属します。 下の図では、横方向の移動が水酸基の付加、縦方向がメチル基の付加にあたります。 |
| Biosynthesis (continued from the chart in Flavonoid) | ||||||
|---|---|---|---|---|---|---|
| (red or orange) | (blue or magenta) | (blue or purple) | ||||
| pelargonidin |
B-ring hydroxylation |
cyanidin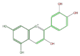
|
B-ring hydroxylation |
delphinidin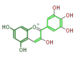
| ||
peonidin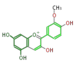
|
petunidin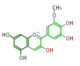
| |||||
|
||||||
malvidin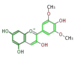
| ||||||
|
All three classes exist in most angio- and gymnosperm orders, and hydroxylating enzymes (F3'H and F3'5'H) show high sequence homology throughout orders. However, genetic changes cause genus-specific inactivation of these pathways. |
3つの経路は殆どの被子、裸子植物目に存在し、水酸化酵素(F3'H と F3'5'H)は高い配列相同性を示します。しかし、種特異的な遺伝子変異のために特定の経路が遮断される場合もあります。 |
| Genus (Family) | Inactivation | Cause |
|---|---|---|
| Arabidopsis (Brassicaceae) Petunia (Solanaceae) Cymbidium (Orchidaceae) |
pelargonidin | DFR does not metabolize dihydrokaempferol. |
| Chrysanthemum (Asteraceae) Dianthus (Caryophyllaceae) Ipomoea (Convolvulaceae) |
delphinidin | F3'5'H is absent. |
構造的特徴
|
Most anthocyanins in autumn leaves are comparatively simple and 3-O-glycosilated (glucoside, galactoside, rhamnoside, gentiobiosid and rutinoside) and/or acylated (p-coumaric, caffeic, ferulic, gallic, acetyl, malonic, malic etc.). Those in storage organs often show complicated structures (see Ipomoea and Dioscorea). |
紅葉に含まれる多くのアントシアニンは多くが単純な構造を持ち3位に糖(グルコース、ガラクトース、ラムノース、ゲンチオビオース、ルチン)、更にアシル化(p-クマル酸、ケイ皮酸、フェルラ酸、没食子酸、酢酸、マロン酸、リンゴ酸)をうけます。芋のような貯蔵器官に含まれるものは複雑な構造が多くなります( サツマイモ属やヤマノイモ属を参照のこと)。 |
データベース統計
主な植物科
|
|
The number in each family is counted as the number of genera (not species) listed in our registered references. Each reference record is accessible by clicking the link in compound pages. The taxonomy follows the APG-II classification. For details (or if the figure is broken), visit this page. 各科のカウントは種名でなく文献に記載された属名の数です。文献は代謝物ページのリンクからたどれ、分類はAPG-IIです。左の図が表示されない場合はここをクリックしてください。 |