Category:FL
m |
|||
| Line 42: | Line 42: | ||
|FL7A||[[:Category:FL7A|Anthocyanin]]<br>[[Image:Fl7.png|100px]] | |FL7A||[[:Category:FL7A|Anthocyanin]]<br>[[Image:Fl7.png|100px]] | ||
|- | |- | ||
| − | |FL7D||[[:Category:FL7D|3-Desoxyanthocyanin]]<br>[[Image:Fl7d.png| | + | |FL7D||[[:Category:FL7D|3-Desoxyanthocyanin]]<br>[[Image:Fl7d.png|125px]] |
|- | |- | ||
|rowspan="10"|FLI | |rowspan="10"|FLI | ||
Revision as of 14:26, 7 March 2008
FL: Flavonoid
Structural Characteristics
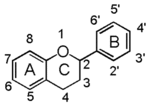
Flavonoid is a class of plant secondary metabolites that have two benzene rings (each called A-ring and B-ring) connected by a chain of three carbons (Figure 1).The carbon chain, corresponding to the numbers 2,3,4 in Figure 1, is linked to a hydroxyl group in the A-ring to form the C-ring. The class of flavonoids are usually determined by the modification pattern of the C-ring (Table 1).
| 1st Class | 2nd Class | ||
|---|---|---|---|
| FL1 | Aurone and Chalcone
|
FL1A | Aurone
|
| FL1B | Auronol | ||
| FL1C | Chalcone
| ||
| FL1D | Dihydrochalcone
| ||
| FL2 | Flavanone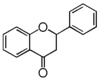 |
FL2F | Flavanone
|
| FL3 | Flavone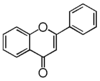 |
FL3F | Flavone
|
| FL4 | Dihydroflavonol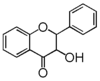 |
FL4D | Dihydroflavonol
|
| FL5 | Flavonol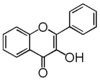 |
FL5F | Flavonol
|
| FL6 | Flavan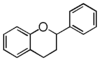
|
FL6F | Flavan
|
| FL63 | Flavan 3-ol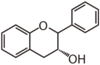
| ||
| FL64 | Flavan 4-ol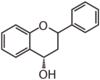
| ||
| FL6D | Flavan 3,4-diol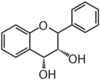
| ||
| FL7 | Anthocyanin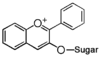
|
FL7A | Anthocyanin
|
| FL7D | 3-Desoxyanthocyanin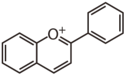
| ||
| FLI | Isoflavonoid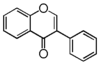
|
FLIA | Isoflavone
|
| FLIB | Isoflavanone
| ||
| FLIC | Isoflavan
| ||
| FLID | Pterocarpane
| ||
| FLIE | Coumestan
| ||
| FLIF | Rotenoid
| ||
| FLIG | Coumaranochromone
| ||
| FLIH | 3-Arylcoumarin
| ||
| FLII | 2-Arylbenzofuran
| ||
| FLIJ | alpha-Methoxynbenzoin
| ||
| FLN | Neoflavonoid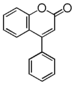
|
FLNA | 4-Arylcoumarin
|
| FLNB | 4-Arylchroman
| ||
| FLNC | Dalbergiquinol
| ||
| FLND | Dalbergione
| ||
| FLNE | Neoflavene
| ||
| FLNF | Coumarinic acid
| ||
Biosynthesis
Flavonoid is synthesized through the phenylpropanoid-acetate pathway in all higher plants. It is responsible for many biological activities including pigments, anti-oxidative or anti-allergic agents, and signaling elements in nodule formation. Some of them are quite familiar in our daily life.
Familiar examples.
anthocyanin (blueberry), isoflavone (soybean), rutin (soba noodle), catechin (tea), flavan-diol (tea), naringeninchalcone (tomato), polyphenol (wine, cacao)
Subcategories
This category has the following 10 subcategories, out of 10 total.