FL63AGNS0001
From Metabolomics.JP
| Flavonoid Top | Molecule Index | Author Index | Journals | Structure Search | Food | New Input |
Upper classes : FL Flavonoid : FL6 Flavan : FL63 Flavan 3-ol : FL63AG Gallocatechin and Epigallocatechin (23 pages) : FL63AGNS Simple substitution (17 pages)
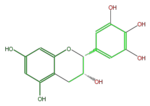
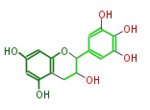
Gallocatechins refer to a subgroup of Flavan 3-ol derivatives (FL63AG). Compared to catechins, they have an additional phenolic hydroxyl group. Catechin gallates are gallic acid esters of the catechins, and (-)-Epigallocatechin gallate (EGCG) is the most abundant catechin in green tea but not in black tea.
(more ...)
| Structure | 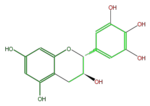
|
|
|
|---|---|---|---|
| Name | (+)-Gallocatechin or D-Gallocatechin; GC |
(-)-Epigallocatechin or L-Epigallocatechin; EGC |
(-)-Gallocatechin or L-Gallocatechin |
| B-ring stereo | ↓ | ↓ | ↑ |
| 3-Hydroxyl stereo | ↑ | ↓ | ↓ |
| Gallate form | 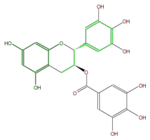
|
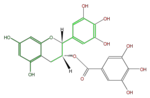
|
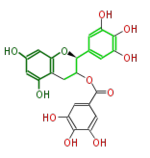
|
| (+)-Gallocatechin gallate or GCG | (-)-Epigallocatechin gallate or EGCG | (-)-Gallocatechin gallate | |
| B-ring stereo | ↓ | ↓ | ↑ |
| 3-Hydroxyl stereo | ↑ | ↓ | ↓ |
| Catechins | (+)-Catechin | (-)-Epicatechin | ent-Catechin |
| IDs and Links | |
|---|---|
| LipidBank | [1] |
| LipidMaps | [2] |
| CAS | 989-51-5 |
| KEGG | {{{KEGG}}} |
| KNApSAcK | |
| CDX file | |
| MOL file | FL63AGNS0001.mol |
| (-)-Epigallocatechin 3-gallate | |
|---|---|
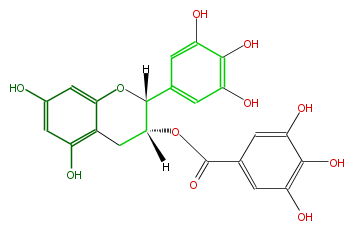
| |
| Structural Information | |
| Systematic Name | (2R-cis) -3,4-Dihydro-5,7-dihydroxy-2- (3,4,5-trihydroxyphenyl) -2H-1-benzopyran-3-yl ester 3,4,5-trihydroxybenzoic acid |
| Common Name |
|
| Symbol | |
| Formula | C22H18O11 |
| Exact Mass | 458.084911418 |
| Average Mass | 458.37172000000004 |
| SMILES | C(c32)C([H])(OC(c(c4)cc(O)c(O)c(O)4)=O)C(Oc(cc(O)c |
| Physicochemical Information | |
| Melting Point | |
| Boiling Point | |
| Density | |
| Optical Rotation | |
| Reflactive Index | |
| Solubility | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | |
| Chromatograms | |
Species Information
| Species-Flavonoid Relationship Reported | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|