Category:FL7
Anthocyanidin, Anthocyanin
| Flavonoid Top | Molecule Index | Author Index | Journals | Structure Search | Food | New Input |
Upper classes : FL Flavonoid
| 2nd Class | |||
|---|---|---|---|
| FL7A | Anthocyanin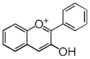
|
FL7D | 3-Deoxyanthocyanin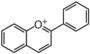
|
Contents |
Overview
Anthocyanin is almost ubiquitous, whereas 3-Deoxyanthocyanin (luteolinidin and apigeninidin) is found only in some lower plants (moss and fern[1][2][3]) and in sorghum (Sorghum bicolor [4]), maize (Zea mays), and gloxinia (Sinningia cardinalis).
The nine known families without anthocyanin are Aizoaceae, Amaranthaceae, Basellaceae, Cactaceae, Chenopodiaceae, Didiereaceae, Nyctaginaceae, Phytolaccaceae, Portulacaceae (all in the order Caryophylalles). These families synthesize the betalain instead of anthocyanin.[5]
The Greek word origin of anthocyanin is "flower" ἀνθός (anthos) and "blue" κυανός (kyanos). It was first used by Marquart to describe water-soluble pigments in red, blue, and purple from flowers [6]. Nowadays, such water-soluble pigments from flowers and fruits are called anthocyan. The flavonoid backbone without sugar (shown below) is called anthocyanidin (it is called aglycon because it is devoid of glyco moiety), and structure with sugars is called anthocyanin. Except for carotenoid (yellow) or indigo (deep blue), most pigments of plant origin are anthocyanins.
- ↑ Bendz G, Martensson O, Terenlus L: Moss pigments I. The anthocyanins of Bryum cryophilum O. Mart. Acta Chem Scand 1962 16:1183-1190
- ↑ Bendz G, Martensson O: Moss pigments II. The anthocyanins of Bryum rutilans Brid. and Bryum weigelii Spreng. Acta Chem Scand 1963 17:266
- ↑ Comparative biochemistry of the flavonoids-II. 3-Desoxyanthocyanins and their systematic distribution in ferns and gesnerads. Phytochemistry 1966 5:589-600
- ↑ Nip WK, Burns EE: Pigment characterization in grain Sorghum. Cereal Chem 1969 46:490-495 also in 1971 48:74-80
- ↑ Piattelli M, Minale L: Pigments of centrospermae-II. Distribution of betacyanins. Phytochemistry 1964 3:547-557
- ↑ Marquart LC. "Die Farben der Bluethen. Eine chemisch-physiol." Abhandlung, Bonn, 1835 (Cited by Onslow MW. "The Anthocyanin Pigments of Plants," Cambridge University Press, 1925).
Target of fruit and flower color engineering
Anthocyanin contains 3 aromatic rings, and glycosylation at the 3-OH position is necessary for stabilizing the aromatic ring. The general color of anchocyanin is red in acidic environment and purple/blue in alkali. The colors are, however, dependent on many other factors such as additional modifications and metal ions as is suggested by multi-color flowers such as pansy (yellow, orange, purple, violet, deep blue).
References
- Kroon J. et al. Plant J. 1994 Jan;5(1):69-80 PMID 8130799
- Boss PK. et al. Plant Mol Biol. 1996 Nov;32(3):565-9 PMID 8980508
- Fukuchi-Mizutani M. et al. Plant Physiol. 2003 Jul;132(3):1652-63 PMID 12857844 (research paper on Blue Rose)
Biodiversity
The six common anthocyanidins are the product of three different branches.
| Biosynthesis (continued from the chart in Flavonoid) | ||||||
|---|---|---|---|---|---|---|
| (red or orange) | (blue or magenta) | (blue or purple) | ||||
| pelargonidin | hydroxylation |
cyanidin | hydroxylation |
delphinidin | ||
| peonidin | petunidin | |||||
|
||||||
| malvidin | ||||||
All three classes exist in most angio- and gymnosperm orders, and hydroxylating enzymes (F3'H and F3'5'H) show high sequence homology throughout orders. However, genetic changes cause genus-specific inactivation of these pathways.
| Genus (Family) | Inactivation | Cause |
|---|---|---|
| Arabidopsis (Brassicaceae) Petunia (Solanaceae) Cymbidium (Orchidaceae) |
pelargonidin | DFR does not metabolize dihydrokaempferol. |
| Chrysanthemum (Asteraceae) Dianthus (Caryophyllaceae) Ipomoea (Convolvulaceae) |
delphinidin | F3'5'H is absent. |
Database statistics データベース統計