Licoricesaponin D3
From Metabolomics.JP
Upper classes
| IDs and Links | |
|---|---|
| LipidBank | [1] |
| LipidMaps | [2] |
| CAS | 118536-87-1 |
| KEGG | {{{KEGG}}} |
| KNApSAcK | |
| CDX file | |
| MOL file | Licoricesaponin D3.mol |
| Licoricesaponin D3 | |
|---|---|
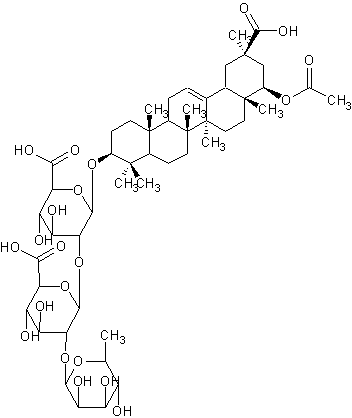
| |
| Structural Information | |
| Systematic Name | (3beta,20beta,22beta)-22-(Acetyloxy)-20-carboxy-30-norolean-12-en-3-yl O-6-deoxy-alpha-L-mannopyranosyl-(12)-O-beta-D-glucopyranuronosyl-(12)-beta-D-glucopyranosiduronic acid |
| Common Name |
|
| Symbol | |
| Formula | C50H76O21 |
| Exact Mass | 1012.487909494 |
| Average Mass | 1013.1258399999999 |
| SMILES | C(O7)(C(OC(O8)C(O)C(C(O)C8C)O)C(C(O)C7C(O)=O)O)OC( |
| Physicochemical Information | |
| Melting Point | |
| Boiling Point | |
| Density | |
| Optical Rotation | |
| Reflactive Index | |
| Solubility | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | |
| Chromatograms | |
Spectroscopic Data
| IR (KBr) | 3700-3200 (br, OH), 2940, 1730 (ester), 1712 (COOH), 1410, 1065 cm-1 |
| 1H-NMR (C5D5N-D2O, 500 MHz) | 0.89 (s, CH3), 0.91 (s, CH3), 0.99 (s, CH3), 1.13 (s, CH3), 1.21 (s, CH3), 1.28 (s, CH3), 1.38 (s, CH3), 1.79 (d, J=5.7 Hz, 3xH-6 of Rha), 2.16 (s, OCOCH3), 3.28 (dd, J=4.2, 11.0 Hz, H-3alpha), 4.59 (dd, J=2.6, 3.4 Hz, H-22), 5.02 (d, J=7.4 Hz, H-1 of GlcUA I), 5.34 (d, J=7.6 Hz, H-1 of GlcUA II), 5.44 (brs, H-12), 6.30 (brs, H-1 of Rha) |
| M.P. | 221 - 223 °C |
| 13C-NMR (C5D5N, 22.5MHz) | C-3) 89.9, (11) 23.6, (12) 122.2, (13) 143.6, (18) 44.0, (22) 77.5, (24) 16.4, (29) 29.1, (30) 177.2, (OCOCH3) 21.8, (OCOCH3) 170.1, (COOCH3) 51.2, 51.2, 51.6 GlcUA I (1) 104.7, (2) 79.1, (3) 76.2, (4) 72.2, (5) 77.9, (6) 169.6 GlcUA II (1)102.4, (2) 78.2, (3) 76.7, (4) 72.9, (5) 77.5, (6) 169.8 Rha (1) 101.6, (2) 71.9, (3) 72.9, (4) 73.9, (5) 69.2, (6) 18.5 |
M. Yoshikawa et al., Chem.Pharm.Bull., 41, 1337 (1993).