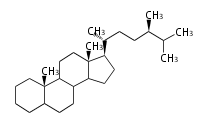
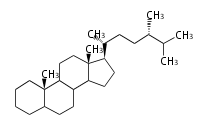
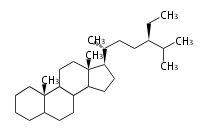
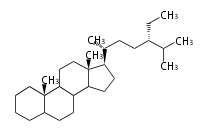
Category:TP3
Contents |
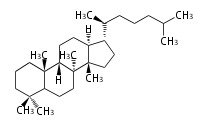
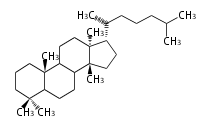
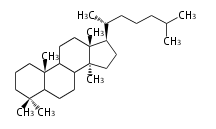
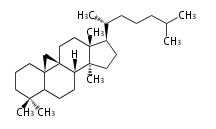
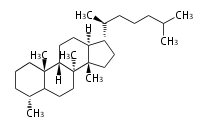
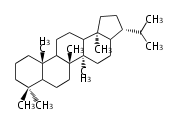
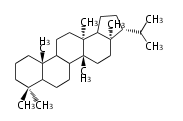
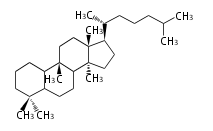
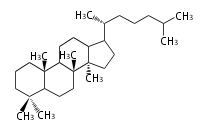
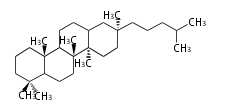
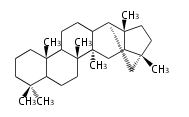
Triterpene (C30)
Ring configuration
Steroid
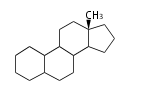
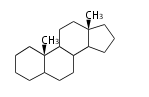
The basic structure is 4 carbon rings, cyclopenta[a]phenanthrene, gonane, or sterane. The rings B/C are always trans in all natural steroids. If the rings C/D are trans, it is called gonane. If its stereochemistry is unspecified, it is called sterane. Most steroids take gonane form, but in cardenolides and bufanolides, the rings C/D are cis.
 |
|
| Cyclopenta[a]phenanthrene | Gonane |
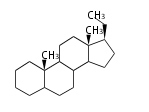
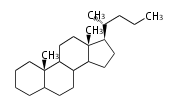
The majority of steroids have methyl groups sticking out from the bridgehead positions C-10 and C-13. When these methyl groups (or hydrogens) stand above the plane, they are called β-configuration. Those below the plane are called α-configuration. If the configuration at any site is unknown, it is indicated as ξ (Greek Xi). By default, hydrogen atoms or substituents at the positions C-8, 9, 10, 13, and 14 are assumed to be 8β, 9α, 10β, 13β, and 14α configurations. C-5 is a special position, because there are as many 5α steroids as 5β are.
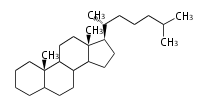 |
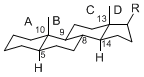 |
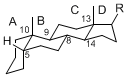
|
| cholestane backbone | 5α-configuration | 5β-configuration |
Triterpenes
In almost all pentacyclic triterpenes in angiosperms, the methyl group at the DE-ring fusion is β-configuration. Some triterpenes in ferns, mosses, gymnosperms have α-methyl group at the DE-ring fusion.
Biosynthesis
Overview
The starting point is squalene, which is formed by joining two FPPs tail-to-tail. Bacterial cyclases use squalene directly[1], but those of the other species use 2,3-oxidosqualene for cyclization.
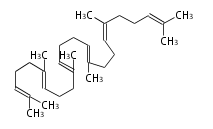
|
|
| squalene | 2,3-oxidosqualene |
- In bacteria, squalene is cyclized via the 17α-deoxydammarenyl cation to hopene and other triterpenes.
- In eukaryotes, 2,3-oxidosqualene is cyclized via the protosteryl cation to lanosterol (animals and fungi), cycloartenol (plants) or parkeol (sea cucumbers) by a series of 1,2-hydride and methyl shifts (Wagner-Meerwein shifts).
- In plants, a trace amount of phytosterols comes from lanosterol [2] At3g45130 is lanosterol synthase in Arabidopsis and its orthologs exist in asterids Taraxacum officinale and Panax ginseng and eurosid Luffa cylindrica. Lanosterol synthase exists broadly among eudicots [3]. Parkeol is also widespread in plants.
- In plants, various triterpenes arise from the 17β-dammarenyl cation.
- References
- ↑ Bacterial squalene cyclases can accept oxidosqualene as their substrates, but oxidosqualene usually does not exist in bacteria
- ↑ Ohyama K, Suzuki M, Kikuchi J, Saito K, Muranaka T (2009) Dual biosynthetic pathways to phytosterol via cycloartenol and lanosterol in Arabidopsis Proc Natl Acad Sci USA 106(3):725-730
- ↑ Kolesnikova MD, Xiong Q, Lodeiro S, Hua L, Matsuda SPT (2006) Lanosterol biosynthesis in plants Arch Biochem Biophys 447:87-95
Oxidosqualene Cyclase in Eukaryotes
Any path of reactions from the root (2,3-oxidosqualene) to any triterpene backbone with a colored background is catalyzed by a single enzyme called oxidosqualene cyclase (OSC) or terpene synthase h (tpsh).[1]
| Backbone Color Code: | Animals, fungi, and yeast | Plants only | |
|---|---|---|---|
| Six-membered rings: | chair (C), or boat (B) | ||
| 2,3-oxidosqualene | |||||||||||||||||

| |||||||||||||||||
|
| ||||||||||||||||
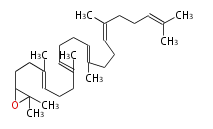
17β-protosteryl cation (C-B-C)[5] 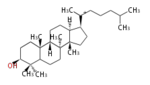
|
1,2-shift |
lanosteryl cation (C-B-C)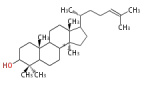
|
17β-dammarenyl cation (C-C-C)[6] 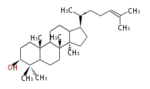
| ||||||||||||||
|
|
| |||||||||||||||
cation with the chain at C18 or C17 position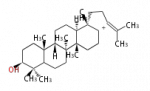 or or 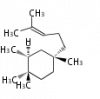
|
|
|
baccarenyl cation (C-C-C-C)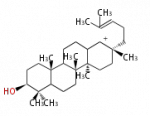
| ||||||||||||||
|
|
|
| ||||||||||||||
arborinyl cation (C-B-C-C)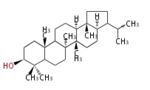
|
unnamed cation (C-B-C-C)
|
21α-hopyl cation (C-C-C-C) 21β-moretyl cation (C-C-C-C)[10] 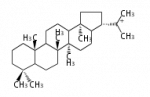
|
H18α-lupyl cation (C-C-C-C) H18β-lupyl cation (C-C-C-B) 
| ||||||||||||||
|
|
|
| ||||||||||||||
| arborinane (C-B-C-C) stictane (C-B-C-C-C)[11] |
hancokinane (C-B-C-C) | hopane (C-C-C-C) gammacerane (C-C-C-C-C) fernane (C-C-C-C) swertane (C-C-C-C-C) |
oleanane[12] (C-C-C-C-C) lupane (C-C-C-C) germanicane taraxastane (C-C-C-C-C) ursane (C-C-C-C-C/B) friedomadeirane (C-C-C-C)[13] | ||||||||||||||
| References |
|---|
|
| Reviews |
|
Squalene Cyclase in Bacteria and Ferns
Squalene cyclase (SC) or terpene synthase g (tpsg) are found in prokaryotes, ciliates, and lower plants (mosses and ferns) and can convert squalene, which is symmetric, as well as 2,3-oxidosqualene. Main products are hopanol and tetrahymanol, and only generate all-chair cations.
squalene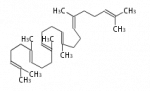
|
squalene-hopene cyclase[1] |
17α-deoxydammarenyl cation[2] 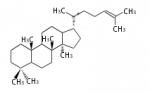
| |||
| |||||
hopene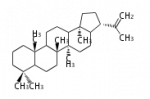
|
|
hopyl cation (c-c-c-c)
|
Hopanoids are widespread: they are found in bacteria, ferns, and geological sediments. They are not found, however, in archaea or animals.
Almost all mono-, di-, tri- and tetracyclic terpenes from squalene are found in ferns: Polypodium, Lemmaphyllum, and Pyrrosia. Notable exception is Z-Dammara-17(20),24-diene from moss Floribundaria[3]. Pentacyclic terpenes from squalene, on the other hand, are found also in angiosperms such as Achillea, Erysimum, Castanopsis and in Ascomycota.
- ↑ SH cyclase is the most investigated enzyme among squalene cyclases.
Ref. Kannenberg EL, Poralla K (1999) Hopanoid biosyntehsis and function in bacteria. Naturwissenschaften 86:168-76. - ↑ The C-17 chain of deoxydammarenyl cation is α-configuration, not β as in eukaryotes.
Ref. Wendt KU, Schulz GE, Corey EJ, Liu DR (2000) Enzyme mechanisms for polycyclic triterpene formation. Angew Chem, Int Ed 39:2812-33 - ↑ Toyota M, Masuda K, Asakawa Y (1998) Triterpenoid constituents of the moss Floribundaria aurea subsp. nipponica. Phytochemistry 48:297–299
Bis-oxidosqualene Cyclase
Squalene is oxidized by squalene oxidase to become 2,3-oxidosqualene. Further epoxidation of this symmetric molecule produces 2,3-(S)-22,23-(S)-bis-oxidosqualene, which is converted to 24,25-epoxylanostan-3-ol or 24,25-epoxycycloartan-3-ol. Epoxylanosterol is known to negatively regulate sterol biosynthesis[1].

|

|
| 2,3-oxidosqualene | 2,3-22,23-bis-oxidosqualene |
- ↑ Gardner RG, Shan H, Matsuda SPT, Hampton RY (2001) A positive oxysterol-derived signal for 3-hydroxy-3-methylglutaryl CoA reductase degradation in yeast. J Biol Chem 276:8681–869
Design of Tri-terpene ID numbers
12-DIGIT
| T | P | 3 | x | y | y | r | h | g | n | c | c |
- x ... species information
| Symbol at x | Kingdom | Phyla | Examples |
|---|---|---|---|
| I | Animalia | Arthropoda (Insects, crabs) | ecdysteroids |
| V | Chordate (Vertebrates) | sex steroids, corticosteroids, anabolic steroids | |
| O | Others | marine steroids | |
| P | Plantae | Phytosterols | lanosterols, cholesterols, brassinolides |
| S | Saponins | saponins | |
| F | Fungi | ergosterols | ergosterols |
| B | Bacteria | bacterial sterols | hopanoids |
- y ... backbone structure (母核構造)
- r ... number of major rings (環構造数)
Click above categories to see details.
- h ... hydroxylation pattern (水酸基数)
Click above categories to see details.
- g ... glycosylation pattern(糖修飾パターン)
Click above categories to see details.
- n ... number of sugars (修飾糖数)
Click above categories to see details.
- c ... serial number (通し番号)
This category currently contains no pages or media.