Category:TP3P
(→{{Bilingual|Do plants contain animal sterols?|植物は動物ステロールも作る}}) |
(→Brassinolides (C28)) |
||
| Line 91: | Line 91: | ||
}} | }} | ||
| − | + | ==Brassinolides (C28)== | |
{{Twocolumn| | {{Twocolumn| | ||
| − | Brassinolides are plant growth-promoting hormones isolated originally from [[Species:Brassica|''Brassica napus'']] (rape). Its backbone is a highly oxygenated ergostane with the oxygen-expanded B-ring (ε-lactone). This lactone is not essential for plant growth activity (e.g. castasterone) but the 22''R'', 23''R''-diol are. | + | Brassinolides are plant growth-promoting hormones isolated originally from [[Species:Brassica|''Brassica napus'']], [[:Category:Brassicaceae|Brassicaceae]] (rape). Its backbone is a highly oxygenated ergostane with the oxygen-expanded B-ring (ε-lactone). This lactone is not essential for plant growth activity (e.g. castasterone) but the 22''R'', 23''R''-diol are. |
The configurations of C-2,3 and 5 are α in brassinolides whereas they are β in ecdysteroids. | The configurations of C-2,3 and 5 are α in brassinolides whereas they are β in ecdysteroids. | ||
| | | | ||
| − | + | ブラシノライドは、植物の成長ホルモンの一つでナタネ ([[Species:Brassica|''Brassica napus'']], [[:Category:Brassicaceae|Brassicaceae]]) で最初に見つかりました。 | |
| + | エルゴスタンが多く酸化された骨格をもち、酸素を含んだB環 (ε-ラクトン) が特徴です。 | ||
| + | このラクトン構造は植物の成長作用に関係ありませんが、22''R'', 23''R''にある二つの水酸基は重要です。 | ||
| + | C-2,3,5位の置換基は、ブラシノライドではα位をとり、エクジステロイドではβ位をとります。 | ||
}} | }} | ||
Revision as of 16:23, 3 August 2010
Contents |
Phytosterols
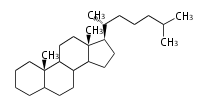
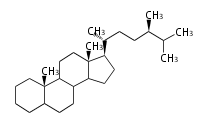
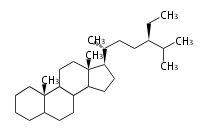
Phytosterols contain extra methyl or ethyl group in the C-17 side chain.
Most common phytosterols are campesterol, β-sitosterol, and stigmasterol. They occur both free and as 3-glucosides and all essential for membranes in higher plant. Less common are α-spinasterol (an isomer of stigmasterol) in spinach (Spinacia oleracea L., Chenopodiaceae) and alfalfa (Medicago sativa L., Fabaceae).
Some sterols are confined in lower plants. Ergosterols are found only in yeast and fungi, fucosterols are found in brown algae (Phaeophyceae) and in some higher plants, e.g. coconut (Cocos nucifera, Arecaceae.
| animal sterols | plant sterols | |
|---|---|---|
 |
 |
|
| cholestane (cholesterol backbone) |
campestane (campesterol backbone) |
stigmastane (β-sito and stigmasterol backbone) |
植物ステロールと健康
Soybean (Glycine max, Fabaceae) is a rich source of phytosterols (about 0.1% of its weight), and is used for semi-synthesis of medicinal steroids [1]. Since dietary phytosterols reduce cholesterol levels, they are used as food additives such as for margarine [2]. Vitamin D is a family of sterol metabolites generated photochemically in our skin by UV irradiation.
- ↑ Yamaya A, Endo Y, Fujimoto K, Kitamura K. (2006) “Effects of genetic variability and planting location on the phytosterol content and composition in soybean seeds” Food Chem 102(4): 1071-1075
- ↑ Schiepers OJ, de Groot RH, van Boxtel MP, Jolles J, de Jong A, Lütjohann D, Plat J, Mensink RP (2009) “Consuming functional foods enriched with plant sterol or stanol esters for 85 weeks does not affect neurocognitive functioning or mood in statin-treated hypercholesterolemic individuals” J Nutr 139(7):1368-1373
植物は動物ステロールも作る
Date palm seed (Phoenix dactylifera, Arecaceae) contains cholesterol and a trace amount of estrone (a female sex hormone). Estrone is also contained in seeds of pomegranate (Punica granatum, Punicaceae). The pollen of Scots pine (Pinus sylvestris, Pinaceae) contains testosterone, the male sex hormone. A large number of ecdysones (insect moulting hormones) have been found in plants, and are called phytoecdysones. Examples are:
- ecdysone from Lychnis fulgens, Caryophyllaceae and many ferns
- ajugasterone from Ajuga chamaepitys and A. reptans, Lamiaceae
- 20-hydroxyecdysone from Serratula inermis, Asteraceae and Diploclisia glaucescens, Menispermaceae).
Cardenolides (C23)
The backbone of the foxglove Digitalis (Plantaginaceae) toxins with the androstane skeleton with a γ-lactone ring at C-17. Notable characters are its 14β-configuration in opposition to other steroids (the rings C/D are cis), and the 20(22)-double bond.
Since Withering's report in 1785 [1], cardenolides have been extremely valuable clinically for congestive heart failure. However, they are remarkably nontoxic to Lepidoptera, especially some species of Danaid butterfly [2]
- ↑ Tröhler U (2007) Withering's 1785 appeal for caution when reporting on a new medicine. J R Soc Med 100(3): 155–156
- ↑ Mebs D, Reuss E, Schneider M (2005) "Studies on the cardenolide sequestration in African milkweed butterflies (Danaidae)" Toxicon 45(5):581-584
Distribution
Cardenolides occur principally in the related families of Apocynaceae (including Asclepiadaceae). In the milkweed genus Asclepias, these compounds are secreted in the latex and almost every species contains these toxins. Other plant families such as Brassicaceae, Moraceae, Scrophulariaceae, and some monocotyledons (e.g. Urginea in Liliaceae) contain cardenolides.
The 5α-configuration is found in Asclepias (e.g. aspecioside), whereas 5β-configuration is common in the foxglove Digitalis purpurea, Plantaginaceae (e.g. digoxin). In some Asclepias, sugars are attached to the two hydroxyl groups at C-2 and C-3 position to form cyclic bridges (e.g. asclepin, calactin).
Withanolides (C28)
Withanolides were found in the root of Withania Somnifera, also known as Indian ginseng. Its backbone is a highly oxygenated ergostane with a γ-lactone ring linking C-22 and C-26 [1]. The configuration of C-22 is usually R.
Brassinolides (C28)
Brassinolides are plant growth-promoting hormones isolated originally from Brassica napus, Brassicaceae (rape). Its backbone is a highly oxygenated ergostane with the oxygen-expanded B-ring (ε-lactone). This lactone is not essential for plant growth activity (e.g. castasterone) but the 22R, 23R-diol are. The configurations of C-2,3 and 5 are α in brassinolides whereas they are β in ecdysteroids.
Cite error:
<ref> tags exist, but no <references/> tag was found
This category currently contains no pages or media.