Category:TP3
Contents |
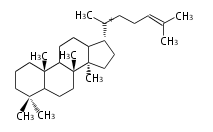
Triterpene (C30) Classes
Ring configuration
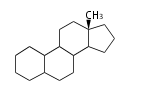
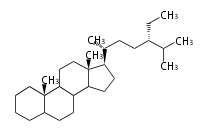
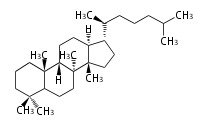
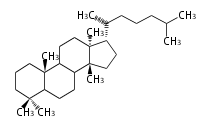
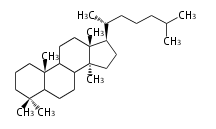
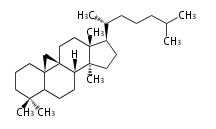
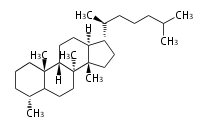
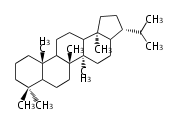
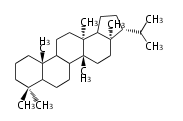
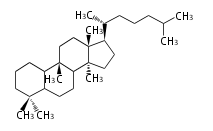
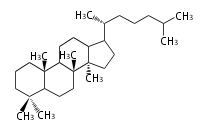
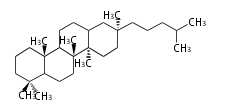
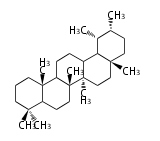
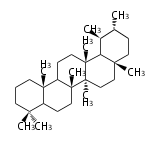
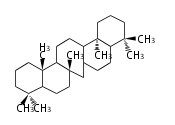
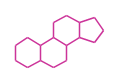
The basic structure is 4 carbon rings, cyclopenta[a]phenanthrene, gonane, or sterane. The rings B/C are always trans in all natural steroids. If the rings C/D are trans, it is called gonane. If its stereochemistry is unspecified, it is called sterane. Most steroids take gonane form, but in cardenolides and bufanolides, the rings C/D are cis.
 |
|
| Cyclopenta[a]phenanthrene | Gonane |
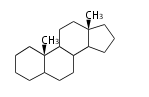
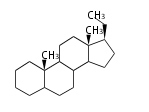
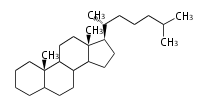
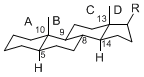
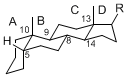
The majority of steroids have methyl groups sticking out from the bridgehead positions C-10 and C-13. When these methyl groups (or hydrogens) stand above the plane, they are called β-configuration. Those below the plane are called α-configuration. If the configuration at any site is unknown, it is indicated as ξ (Greek Xi). By default, hydrogen atoms or substituents at the positions C-8, 9, 10, 13, and 14 are assumed to be 8β, 9α, 10β, 13β, and 14α configurations. C-5 is a special position, because there are as many 5α steroids as 5β are.
 |
 |
|
| cholestane backbone | 5α-configuration | 5β-configuration |
Biosynthesis
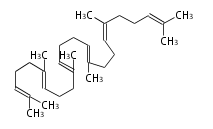
The starting point is squalene, which is formed by joining two FPPs tail-to-tail. Bacterial cyclases use squalene directly[1], but those of the other species use 2,3-oxidosqualene for cyclization.
- In bacteria, squalene is cyclized via the 17α-deoxydammarenyl cation to hopene and other triterpenes.
- In eukaryotes, 2,3-oxidosqualene is cyclized via the protosteryl cation to lanosterol (animals and fungi) or cycloartenol (plants) by a series of 1,2-hydride and methyl shifts (Wagner-Meerwein shifts).[2]
- In plants, various triterpenes arise from the 17β-dammarenyl cation.
真核生物
| (骨格色分け: | Animals, fungi, and yeast | Plants only | ) |
|---|
| 2,3-oxidosqualene | ||||||||||||||||||
| ||||||||||||||||||
|
| |||||||||||||||||
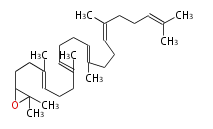
17β-protosteryl cation (C-B-C)[5] 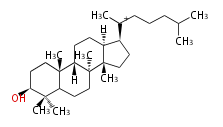
|
1,2-shift |
lanosteryl cation (C-C-C)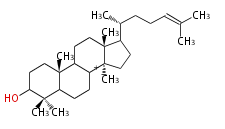
|
17β-dammarenyl cation (C-C-C)[6] 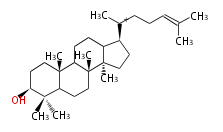
| |||||||||||||||
|
|
|
||||||||||||||||
cation with the chain at C18 or C17 position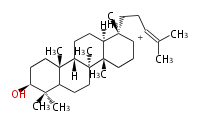 or or 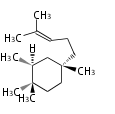
|
|
|
baccarenyl cation (C-C-C-C)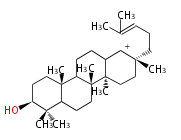
| |||||||||||||||
|
|
|
| |||||||||||||||
arborinyl cation (C-B-C-C)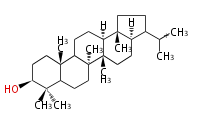
|
unnamed cation (C-B-C-C)
|
21α-hopyl cation (C-C-C-C) 21β-moretyl cation (C-C-C-C)[10] 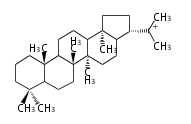
|
H18α-lupyl cation (C-C-C-C) H18β-lupyl cation (C-C-C-B) 
| |||||||||||||||
|
|
|
| |||||||||||||||
| arborinane (C-B-C-C) stictane (C-B-C-C-C)[11] |
hancokinane (C-B-C-C) | hopane (C-C-C-C) gammacerane (C-C-C-C-C) fernane (C-C-C-C) swertane (C-C-C-C-C) |
oleanane (C-C-C-C-C) lupane (C-C-C-C) germanicane taraxastane (C-C-C-C-C) ursane (C-C-C-C-C/B) friedomadeirane (C-C-C-C)[12] | |||||||||||||||
- ↑ Bacterial squalene cyclases can accept oxidosqualene as their substrates, but oxidosqualene usually does not exist in bacteria
- ↑ In plants, a trace amount of phytosterols comes from lanosterol.
Ref. Ohyama K, Suzuki M, Kikuchi J, Saito K, Muranaka T (2009) Dual biosynthetic pathways to phytosterol via cycloartenol and lanosterol in Arabidopsis Proc Natl Acad Sci USA 106(3):725-730 - ↑ Lanosterol synthase is the most accessible enzyme among oxidosqualene cyclases.
Ref. Corey EJ, Russey WE, Ortiz-de-Montellano PR (1966) 2,3-Oxidosqualene, an intermediate in the biological synthesis of sterols from squalene J Am Chem Soc 88:4750-1 - ↑ The cyclization process is stepwise, not concerted as previously thought. As one clue, squalene is not fully folded in the cyclase active site.
Ref. Reinert DJ, Balliano G, Schulz GE (2004) Conversion of squalene to the pentacarbocyclic hopene. Chem Biol 11:121-6 - ↑ The C-17 chain of rotosteryl cation is β-configuration, not α.
Ref. Corey EJ, Virgil SC (1991) An experimental demonstration of the stereochemistry of enzymic cyclization of 2,3-oxidosqualene to the protosterol system, forerunner of lanosterol and cholesterol. J Am Chem Soc 113:4025-6 - ↑ The C-17 chain of dammarenyl cation is β-configuration.
Ref. Xiong Q, Rocco F, Wilson WK, Xu R, Ceruti M, Matsuda SPT (2005) Structure and reactivity of the dammarenyl cation: configuration transmission in triterpene synthesis. J Org. Chem. 70:5362-75 - ↑ Oxygenated protostane are known in Cephalosporium caerulens, Fusidium coccineum, and Aspergillus fumigatus (Ascomycota).
Ref. Hattori H, Igarashi H, Iwasaki S, Okuda S, (1969) Isolation of 3bhydroxy- 4b-methylfusida-17(20)[16,21-cis],24 diene (3b-hydroxyprotosta- 17(20)[16,21-cis],24 diene) and a related triterpene alcohol Tetrahedron Lett 13, 1023–1026
Tiwari KP, Choudhary RN (1981) Chemical examination of Wisteria sinensis Proc Natl Acad Sci India A 51, 263–271 - ↑ Dammarane derivatives are unusually prevalent in the genus Euphorbia.
- ↑ No 17α cyclization for the ring-B boat form. Also no squalene cyclase is known for the ring-B boat form.
Ref. Xiong Q, Rocco F, Wilson WK, Xu R, Ceruti M, Matsuda SPT (2005) Structure and reactivity of the dammarenyl cation: configurational transmission in triterpene synthesis. J Org Chem 70:5362-75 - ↑ The 21R stereocenter is usually lost in hydride shift.
- ↑ C-B-C-C-C rings are very rare and stictanediol from Ascomycota Sticta genus is the sole example.
Ref. Chin WJ, Corbett RE, Heng CK, Wilkins AL (1973) Lichens and fungi. XI. Isolation and structural elucidation of a new group of triterpenes from Sticta coronata, S. colensoi, and S. flavicans. J Chem Soc Perkin Trans 1:1437–46 - ↑ E-ring cyclization precedes D-ring expansion.
Useful Reviews:
- Xu R, Fazio GC, Matsuda SPT (2004) On the origins of triterpenoid skeletal diversity. Phytochemstry 65:261-291 PMID 14751299
微生物
| BACTERIA | |
| squalene | |
|
|
| squalene-hopene |
|
| 17α-deoxydammarenyl cation[2] | |
| D-ring |
|

|
|
| |
|
hopene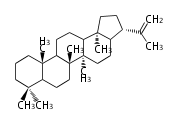
|
|
| Examples. |
Design of Tri-terpene ID numbers ID番号の設計
12-DIGIT
| T | P | 3 | x | y | y | r | h | g | n | c | c |
- x ... species information
| Symbol at x | Kingdom | Phyla | Examples |
|---|---|---|---|
| I | Animalia | Arthropoda (Insects, crabs) | ecdysteroids |
| V | Chordate (Vertebrates) | sex steroids, corticosteroids, anabolic steroids | |
| O | Others | marine steroids | |
| P | Plantae | Phytosterols | lanosterols, cholesterols, brassinolides |
| S | Saponins | saponins | |
| F | Fungi | ergosterols | ergosterols |
| B | Bacteria | bacterial sterols | hopanoids |
- y ... backbone structure (母核構造)
- r ... number of major rings (環構造数)
Click above categories to see details.
- h ... hydroxylation pattern (水酸基数)
Click above categories to see details.
- g ... glycosylation pattern(糖修飾パターン)
Click above categories to see details.
- n ... number of sugars (修飾糖数)
Click above categories to see details.
- c ... serial number (通し番号)
Cite error:
<ref> tags exist, but no <references/> tag was found
This category currently contains no pages or media.