Category:TP3
m (→Steroid) |
m (→{{Bilingual|バクテリア・シダ類のスクアレン環化酵素|Squalene Cyclase in Bacteria and Ferns}}) |
||
| (58 intermediate revisions by one user not shown) | |||
| Line 1: | Line 1: | ||
| − | ==Triterpene (C30) | + | ==Triterpene (C30)== |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | == | + | =={{Bilingual|環の構造|Ring configuration}}== |
| − | === | + | ==={{Bilingual|ステロイド|Steroid}}=== |
{{Twocolumn| | {{Twocolumn| | ||
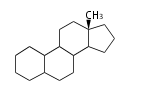
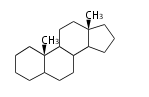
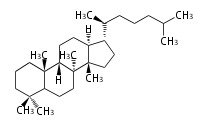
| − | The basic | + | The basic structure is 4 carbon rings, cyclopenta[a]phenanthrene, gonane, or sterane. |
The rings B/C are always ''trans'' in all natural steroids. If the rings C/D are ''trans'', it is called gonane. If its stereochemistry is unspecified, it is called sterane. | The rings B/C are always ''trans'' in all natural steroids. If the rings C/D are ''trans'', it is called gonane. If its stereochemistry is unspecified, it is called sterane. | ||
Most steroids take gonane form, but in cardenolides and bufanolides, the rings C/D are ''cis''. | Most steroids take gonane form, but in cardenolides and bufanolides, the rings C/D are ''cis''. | ||
| | | | ||
| − | + | 基本骨格は4つの環構造で、シクロペンタ[a]フェナンスレン、ゴナン、ステランなどと呼ばれます。 | |
| − | 天然のステロイドでは環 B/C | + | 天然のステロイドでは環 B/C は常にトランスの位置にあります。 |
| − | + | 環 C/D がトランスの場合をゴナン、立体配置が指定されていないときをステランと呼びます。 | |
| + | ほとんどのステロイドはゴナンの形をとりますが、カルデノライドとブファノライドは環 C/D がシスになります。 | ||
}} | }} | ||
| Line 34: | Line 30: | ||
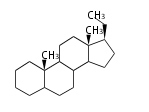
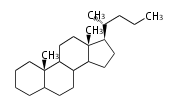
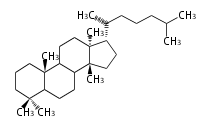
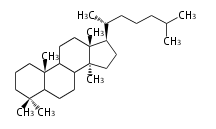
8β, 9α, 10β, 13β, and 14α configurations. C-5 is a special position, because there are as many 5α steroids as 5β are. | 8β, 9α, 10β, 13β, and 14α configurations. C-5 is a special position, because there are as many 5α steroids as 5β are. | ||
| | | | ||
| − | 大多数のステロイドは橋頭位のC-10, C- | + | 大多数のステロイドは橋頭位のC-10, C-13からメチル基が出ます。 |
| − | これらのメチル基 (または水素など) | + | これらのメチル基 (または水素など) が平面より上に出ているときを、ベータ配置とします。平面より下の場合がアルファ配置です。 |
| − | + | 配置が不明な場合は、ξ (ギリシャ語の Xi)で表します。 | |
| − | 原則として、C-8, 9, 10, 13, 14位にある水素や置換基はそれぞれ8β, 9α, 10β, 13β, 14α | + | |
| − | C- | + | 原則として、C-8, 9, 10, 13, 14位にある水素や置換基はそれぞれ8β, 9α, 10β, 13β, 14α 配置です。 |
| + | C-5位だけは特別で、5α のステロイドと 5β のものが同じくらい存在するので指定が必要です。 | ||
}} | }} | ||
<center> | <center> | ||
{| style="text-align:center" | {| style="text-align:center" | ||
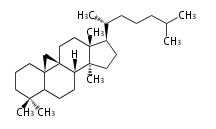
| − | | [[Image:5alpha-steroid.png]] || [[Image:5beta-steroid.png]] | + | | [[Image:cholestane.png]] || [[Image:5alpha-steroid.png]] || [[Image:5beta-steroid.png]] |
|- | |- | ||
| + | | cholestane backbone | ||
| 5α-configuration | | 5α-configuration | ||
| 5β-configuration | | 5β-configuration | ||
| Line 50: | Line 48: | ||
</center> | </center> | ||
| − | === | + | ==={{Bilingual|トリテルペン|Triterpenes}}=== |
| − | + | ||
| − | {{ | + | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | | | + | |
| − | + | ||
| − | }} | + | |
| − | |||
{{Twocolumn| | {{Twocolumn| | ||
| − | + | In almost all pentacyclic triterpenes in angiosperms, the methyl group at the DE-ring fusion is β-configuration. Some triterpenes in ferns, mosses, gymnosperms have α-methyl group at the DE-ring fusion. | |
| − | + | ||
| − | + | ||
| | | | ||
| − | + | 被子植物におけるほとんどの5環テルペンではDE環の継ぎ目にあるメチル基がβ-配置を取ります。シダ類、コケ類、裸子植物における一部の5環テルペンではDE環にα-配置のメチル基がみられます。 | |
}} | }} | ||
| − | ==== | + | =={{Bilingual|生合成|Biosynthesis}}== |
| + | ==={{Bilingual|概要|Overview}}=== | ||
{{Twocolumn| | {{Twocolumn| | ||
| − | The | + | The starting point is squalene, which is formed by joining two FPPs tail-to-tail. |
| − | + | Bacterial cyclases use squalene directly<ref>Bacterial squalene cyclases can accept oxidosqualene as their substrates, but oxidosqualene usually does not exist in bacteria</ref>, but those of the other species use | |
| − | + | 2,3-oxidosqualene for cyclization. | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| | | | ||
| + | 始まりとなる物質はスクアレンで、二分子のファルネシル二リン酸が末尾どうしで結合して作られます。バクテリアの環化酵素はスクアレンをそのまま利用しますが<ref>バクテリアのスクアレン環化酵素はオキシドスクアレンも基質として利用できますが、バクテリアはオキシドスクアレンを合成しません。</ref>、それ以外の生物種では2,3-オキシドスクアレンを利用します。 | ||
}} | }} | ||
| − | + | <center> | |
| − | = | + | {| style="text-align:center" |
| + | | [[Image:squalene.png]] | ||
| + | | [[Image:2,3-oxidosqualene.png]] | ||
| + | |- | ||
| + | | squalene | ||
| + | | 2,3-oxidosqualene | ||
| + | |} | ||
| + | </center> | ||
{{Twocolumn| | {{Twocolumn| | ||
| − | + | * In bacteria, squalene is cyclized via the 17α-deoxydammarenyl cation to hopene and other triterpenes. | |
| − | + | * In eukaryotes, 2,3-oxidosqualene is cyclized via the protosteryl cation to lanosterol (animals and fungi), cycloartenol (plants) or parkeol (sea cucumbers) by a series of 1,2-hydride and methyl shifts (Wagner-Meerwein shifts). | |
| − | * | + | * In plants, a trace amount of phytosterols comes from lanosterol <ref>Ohyama K, Suzuki M, Kikuchi J, Saito K, Muranaka T (2009) Dual biosynthetic pathways to phytosterol via cycloartenol and lanosterol in Arabidopsis ''Proc Natl Acad Sci USA'' 106(3):725-730</ref> '''At3g45130''' is lanosterol synthase in ''Arabidopsis'' and its orthologs exist in asterids [[Species:Taraxacum|''Taraxacum officinale'']] and [[Species:Panax|''Panax ginseng]] and eurosid [[Species:Luffa|''Luffa cylindrica]]. Lanosterol synthase exists broadly among eudicots <ref>Kolesnikova MD, Xiong Q, Lodeiro S, Hua L, Matsuda SPT (2006) Lanosterol biosynthesis in plants ''Arch Biochem Biophys'' 447:87-95</ref>. Parkeol is also widespread in plants. |
| − | * | + | * In plants, various triterpenes arise from the 17β-dammarenyl cation. |
| − | * | + | |
| | | | ||
| − | + | * バクテリアでは、スクアレンが環化して17α-ダンマラン型カチオンとなり、最終的にホペン等のトリテルペンになります。 | |
| + | * 真核生物では、2,3-オキシドスクアレンが環化してプロトステロール型カチオンを作り、プロトンとメチル基の転移 (Wagner-Meerweinシフト) を経て1,2-ラノステロール (動物や真菌類)、シクロアルテノール (植物)や、パルケオール(ナマコ) になります。 | ||
| + | * 植物ではラノステロールからも一部の植物ステロールが合成されます。シロイヌナズナの '''At3g45130''' はラノステロール合成遺伝子であり、オーソログが菊類の [[Species:Taraxacum|''Taraxacum officinale'']] や [[Species:Panax|''Panax ginseng'']]、 真正バラ類の [[Species:Luffa|''Luffa cylindrica'']] にあることから、真正双子葉類に幅広く見られると考えられます。パーケオールも幅広い植物に見られます。 | ||
| + | * 更に植物の場合、17β-ダンマラン型のカチオンから様々なトリテルペンが生成されます。 | ||
}} | }} | ||
| − | + | ;References | |
| + | <references/> | ||
| + | <br/> | ||
| − | === | + | ==={{Bilingual|真核生物のオキシドスクアレン環化酵素|Oxidosqualene Cyclase in Eukaryotes}}=== |
{{Twocolumn| | {{Twocolumn| | ||
| − | + | Any path of reactions from the root (2,3-oxidosqualene) to any triterpene backbone with a colored background is catalyzed by a single enzyme called ''oxidosqualene cyclase'' (OSC) or ''terpene synthase h'' (tpsh).<ref>Terpene synthases a-f are responsible for mono-, sesquie- and diterpenes. Tps g is the squalene cyclase.</ref> | |
| | | | ||
| − | + | この図の最上段 (2,3-オキシドスクアレン) から背景色のついたテルペン骨格までの任意の経路を、オキシドスクアレン環化酵素 | |
| + | (OSC) またはテルペンシンターゼ h (Tps h) と呼ばれる単一の酵素が触媒します。<ref>テルペンシンターゼ a-f は、モノ、セスキ、ジテルペンを合成します。 Tps g はスクアレン環化酵素です。</ref> | ||
}} | }} | ||
| − | + | {| | |
| − | {{ | + | |- |
| − | + | ! {{Bilingual|骨格色分け:|Backbone Color Code:}} | |
| − | + | ! style="background-color:#fdd"| Animals, fungi, and yeast | |
| − | | | + | ! style="background-color:#dfd"| Plants only |
| − | + | ! | |
| + | |- | ||
| + | ! {{Bilingual|6員環表記:|Six-membered rings:}} | ||
| + | ! colspan="2" | chair (C), or boat (B) | ||
| + | |} | ||
| − | === | + | <center> |
| − | + | {|style="text-align:center" | |
| − | ==== | + | |- |
| − | { | + | |colspan="2"| |
| − | + | |colspan="2"| 2,3-oxidosqualene | |
| − | < | + | |- |
| − | + | |colspan="2"| | |
| − | + | |colspan="2"|[[Image:2,3-oxidosqualene.png]] | |
| − | + | |- | |
| − | + | |colspan="2" align="right"| | |
| − | + | {| | |
| − | + | |lanosterol/<br/>cycloartenol<br/>syntase<ref>Lanosterol synthase is the most accessible enzyme among oxidosqualene cyclases, e.g. from mammalian liver or yeast. Cycloartenol synthase is the basic OSC in plants, although lanosterol synthase is also found. For the grouping of cyclases, check the review by Xiong Q et al. (2005) in this page.<br/>''References.'' | |
| − | + | * Corey EJ, Russey WE, Ortiz-de-Montellano PR (1966) 2,3-Oxidosqualene, an intermediate in the biological synthesis of sterols from squalene ''J Am Chem Soc'' 88:4750-1 | |
| − | + | * Kolesnikova MD, Xiong Q, Lodeiro S, Hua L, Matsuda SPT (2006) Lanosterol biosynthesis in plants ''Arch Biochem Biophys'' 447:87-95<br/> | |
| + | In ''Arabidopsis'', cycloartenol synthase can be converted to lanosterol synthase with only two amino acid substitutions: His477 to Asn and Ile481 to Val. Tyr410 is also important for specificity. | ||
| + | * Lodeiro S, Schulz-Gasch T, Matsuda SPT (2005) Enzyme redesign: two mutations cooperate to convert cycloartenol synthase into an accurate lanosterol synthase ''J Am Chem Soc'' 127:14132-14133 | ||
| + | </ref> | ||
| + | |[[Image:Arrow00dl35.png]] | ||
| + | |stepwise<br/>cyclization<ref>The cyclization process is stepwise, not concerted as previously thought. As one clue, squalene is not fully folded in the cyclase active site.<br/>''Ref.'' Reinert DJ, Balliano G, Schulz GE (2004) Conversion of squalene to the pentacarbocyclic hopene. ''Chem Biol'' 11:121-6</ref> | ||
| + | |} | ||
| + | |colspan="2"| | ||
| + | |align="left"| | ||
| + | {| | ||
| + | |lupeol/<br/>β-amyrin<br/>synthase<ref>Lupeol synthases are found in [[Species:Glycyrrhiza|''Glycyrrhiza glabra'']], [[Species:Betula|''Betula'' platyphylla]], [[Species:Taraxacum|''Taraxacum officinale'']], and [[Species:Olea|''Olea europea'']]. They form a clade (74-81% identical) that is distinct from other OSCs. Lupeol synthase evolved before the divergence of asterids and eurosids. On the other hand, β-amyrin synthases are considerably more distant (48-50% identical) than are the CAS enzymes (70-79% identical). β-amyrin synthases in eudicotsa nd monocots may have independent origins.<ul><li>Haralampidis K, Bryan G, Qi X, Papadopoulou K, Bakht S, Melton R, Osbourn A (2001) A new class of oxidosqualene cyclases directs synthesis of antimicrobial phytoprotectants in monocots ''Proc Natl Acad Sci U S A'' 98(23):13431-6 <li>Qi X, Bakht S, Leggett M, Maxwell C, Melton R, Osbourn A (2004) A gene cluster for secondary metabolism in oat: implications for the evolution of metabolic diversity in plants ''Proc Natl Acad Sci U S A'' 101(21):8233-8</ul></ref> | ||
| + | |[[Image:Arrow00dr35.png]] | ||
| + | |stepwise<br/>cyclization | ||
| + | |} | ||
| + | |- | ||
| + | | 17β-protosteryl cation <small>(C-B-C)</small><ref>The C-17 chain of rotosteryl cation is β-configuration, not α.<br/> ''Ref.'' Corey EJ, Virgil SC (1991) An experimental demonstration of the stereochemistry of enzymic cyclization of 2,3-oxidosqualene to the protosterol system, forerunner of lanosterol and cholesterol. [http://pubs.acs.org/doi/abs/10.1021/ja00010a073 ''J Am Chem Soc'' 113:4025-6]</ref> <br/>[[Image:Protosteryl cation.png|150px]] | ||
| + | | 1,2-shift<br/>[[Image:Arrow00r.png]] | ||
| + | | colspan="2" align="left"|lanosteryl cation <small>(C-B-C)</small><br/>[[Image:Lanosteryl cation.png|150px]] | ||
| + | | 17β-dammarenyl cation <small>(C-C-C)</small><ref>The C-17 chain of dammarenyl cation is β-configuration.<br/> ''Ref.'' Xiong Q, Rocco F, Wilson WK, Xu R, Ceruti M, Matsuda SPT (2005) Structure and reactivity of the dammarenyl cation: configuration transmission in triterpene synthesis. ''J Org. Chem. 70:5362-75</ref> <br/>[[Image:Dammarenyl cation.png|150px]] | ||
| + | |- | ||
| + | |colspan="2"| | ||
| + | {| | ||
| | | | ||
| − | + | {| | |
| − | + | |- | |
| − | + | |D-ring<br/>expansion | |
| − | + | |[[Image:Arrow00d.png]] | |
| − | + | |} | |
| − | + | ||
| − | + | ||
| | | | ||
| − | + | {| | |
| − | + | | [[Image:Arrow00dr35.png]] | |
| − | + | | fungus<br/>only<ref>Oxygenated protostane are known in ''Cephalosporium caerulens'', ''Fusidium coccineum'', and ''Aspergillus fumigatus'' (Ascomycota). <br/>''Ref.'' Hattori H, Igarashi H, Iwasaki S, Okuda S, (1969) Isolation of 3bhydroxy- 4b-methylfusida-17(20)[16,21-cis],24 diene (3b-hydroxyprotosta- | |
| − | { | + | 17(20)[16,21-cis],24 diene) and a related triterpene alcohol ''Tetrahedron Lett'' 13, 1023–1026<br/>Tiwari KP, Choudhary RN (1981) Chemical examination of ''Wisteria sinensis'' ''Proc Natl Acad Sci India A'' 51, 263–271</ref> |
| − | + | |- | |
| − | ( | + | | |
| − | <br/> | + | |style="background-color:#fdd" align="right"| protostane |
| − | + | |} | |
| − | + | |} | |
| − | + | | colspan="2"| | |
| + | {| | ||
| + | |1,2-shifts [[Image:Arrow00d.png]] all eukaryotes | ||
| + | |} | ||
| | | | ||
| + | {| | ||
| + | |- | ||
| + | | D-ring<br/>expansion | ||
| + | |[[Image:Arrow00d.png]] | ||
| + | |[[Image:Arrow00dr35.png]]<br/><br/> | ||
| + | |style="background-color:#dfd" align="center"| dammarane<br/>euphane<br/>tirucalane etc.<ref>Dammarane derivatives are unusually prevalent in the genus [[Species:Euphorbia|Euphorbia]].</ref> | ||
| + | |} | ||
| + | |- valign="top" | ||
| + | |colspan="2"| cation with the chain at C18 or C17 position<br/>[[Image:CBCC cation.png|150px]]or [[Image:CBCC cation2.png|100px]] | ||
| + | | | ||
| + | {| | ||
| + | |style="background-color:#fdd"| all steroids<br/>lanostane<br/>cycloartane<br/>cucurbitane<br/>ergostane etc. | ||
| + | |} | ||
| + | |style="vertical-align:bottom"| | ||
| + | {| style="margin-left:auto;margin-right:0;" | ||
| + | |style="background-color:#dfd"| baccharane<br/>shionane | ||
| + | | [[Image:Arrow00l35.png]] | ||
| + | |} | ||
| + | | baccarenyl cation <small>(C-C-C-C)</small><br/>[[Image:Baccarenyl cation.png|150px]] | ||
| + | |- | ||
| + | | | ||
| + | {| | ||
| + | | E-ring<br/>cyclization<br/>(from 17β) | ||
| + | | [[Image:Arrow00d.png]] | ||
| + | | No 17α known<br/>in nature <ref name="JOC">No 17α cyclization for the ring-B boat form. Also no squalene cyclase is known for the ring-B boat form.<br/>''Ref''. Xiong Q, Rocco F, Wilson WK, Xu R, Ceruti M, Matsuda SPT (2005) Structure and reactivity of the dammarenyl cation: configurational transmission in triterpene synthesis. ''J Org Chem'' 70:5362-75</ref> | ||
| + | |} | ||
| + | | | ||
| + | {| | ||
| + | | E-ring<br/>cyclization<br/>(from 18α) | ||
| + | | [[Image:Arrow00d.png]] | ||
| + | |} | ||
| + | | | ||
| + | | | ||
| + | {| style="margin-left:auto;margin-right:0;" | ||
| + | | E-ring cyclization<br/>(from 17α/β) | ||
| + | | [[Image:Arrow00dl.png]] | ||
| + | |} | ||
| + | | | ||
| + | {| | ||
| + | |E-ring cyclization<br/>(from 18α/β) | ||
| + | |[[Image:Arrow00d.png]] | ||
| + | |} | ||
| + | |- valign="top" | ||
| + | | arborinyl cation <small>(C-B-C-C)</small><br/>[[Image:Arborinyl cation.png|150px]] | ||
| + | | colspan="2" align="left"| unnamed cation <small>(C-B-C-C)</small><br/>[[Image:Hanco cation.png|150px]] | ||
| + | | 21α-hopyl cation <small>(C-C-C-C)</small><br/>21β-moretyl cation <small>(C-C-C-C)</small><ref>The 21''R'' stereocenter is usually lost in hydride shift.</ref><br/>[[Image:Hopyl cation.png|150px]] | ||
| + | | H18α-lupyl cation <small>(C-C-C-C)</small><br/>H18β-lupyl cation <small>(C-C-C-B)</small><br/>[[Image:Lupyl cation.png|150px]] | ||
| + | |- | ||
| + | |align="left"| | ||
| + | {| | ||
| + | |1,2-shifts | ||
| + | |[[Image:Arrow00d35.png]] | ||
| + | |E-ring expansion | ||
| + | |} | ||
| + | | | ||
| + | {| | ||
| + | |1,2-shifts | ||
| + | |[[Image:Arrow00d35.png]] | ||
| + | |E-ring expansion | ||
| + | |} | ||
| + | | | ||
| + | | | ||
| + | {| | ||
| + | |1,2-shifts | ||
| + | |[[Image:Arrow00d35.png]] | ||
| + | |E-ring expansion | ||
| + | |} | ||
| + | | | ||
| + | {| | ||
| + | |1,2-shifts | ||
| + | |[[Image:Arrow00d35.png]] | ||
| + | |E-ring expansion | ||
| + | |} | ||
| + | |- | ||
| + | |style="background-color:#dfd"| arborinane (C-B-C-C)<br/>stictane (C-B-C-C-C)<ref>C-B-C-C-C rings are very rare and stictanediol from Ascomycota [[Species:Sticta|''Sticta'']] genus is the sole example.<br/>''Ref.'' Chin WJ, Corbett RE, Heng CK, Wilkins AL (1973) Lichens and fungi. XI. Isolation and structural elucidation of a new group of triterpenes from ''Sticta coronata, S. colensoi'', and ''S. flavicans''. ''J Chem Soc Perkin Trans'' 1:1437–46</ref> | ||
| + | |style="background-color:#dfd"| hancokinane (C-B-C-C) | ||
| + | | | ||
| + | |style="background-color:#dfd" align="left"| hopane (C-C-C-C)<br/> gammacerane (C-C-C-C-C)<br/> fernane (C-C-C-C)<br/> swertane (C-C-C-C-C)<br/> | ||
| + | |style="background-color:#dfd" align="left"| oleanane<ref>β-amyrin has the oleanane skeleton</ref> (C-C-C-C-C)<br/> lupane (C-C-C-C)<br/> germanicane<br/> taraxastane (C-C-C-C-C)<br/> ursane (C-C-C-C-C/B)<br/> friedomadeirane (C-C-C-C)<ref>E-ring cyclization precedes D-ring expansion.</ref><br/> | ||
| + | |} | ||
| − | + | </center> | |
| − | == | + | {| class="wikitable collapsible" style="width:100%" |
| − | + | ! References | |
| − | + | |- | |
| + | | <references/> | ||
| + | |- | ||
| + | ! Reviews | ||
| + | |- | ||
| | | | ||
| − | + | * Xu R, Fazio GC, Matsuda SPT (2004) On the origins of triterpenoid skeletal diversity. ''Phytochemstry'' 65:261-291 PMID 14751299 | |
| + | * Philips DR, Rasbery JM, Bartel B, Matuda SPT (2006) Biosynthetic diversity in plant triterpene cyclization ''Curr Opin Plant Biol'' 9:305-314 | ||
| + | |} | ||
| − | === | + | ==={{Bilingual|バクテリア・シダ類のスクアレン環化酵素|Squalene Cyclase in Bacteria and Ferns}}=== |
| − | {{ | + | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | |||
| − | |||
| − | |||
{{Twocolumn| | {{Twocolumn| | ||
| − | + | Squalene cyclase (SC) or terpene synthase g (tpsg) are found in prokaryotes, ciliates, and lower plants (mosses and ferns) and can convert squalene, which is symmetric, as well as 2,3-oxidosqualene. | |
| − | + | Main products are hopanol and tetrahymanol, and only generate all-chair cations. | |
| + | | | ||
| + | スクアレン環化酵素 (SC) またはテルペン合成酵素 g (tpsg) は原核生物、せん毛虫、下等な植物(コケ・シダ類)に見られ、スクアレンだけでなく2,3-オキシドスクアレンも触媒します。主な代謝産物はホパノールやテトラヒマノールで、全て椅子型の骨格しか生成しません。 | ||
}} | }} | ||
| − | + | {| style="text-align:center" | |
| − | {| style="text-align:center | + | | squalene<br/>[[Image:squalene.png|150px]] |
| − | | | + | | [[Image:Arrow00r.png]]<br/>squalene-hopene cyclase<ref>SH cyclase is the most investigated enzyme among squalene cyclases. <br/>''Ref.'' Kannenberg EL, Poralla K (1999) Hopanoid biosyntehsis and function in bacteria. ''Naturwissenschaften'' 86:168-76.</ref> |
| − | + | | 17α-deoxydammarenyl cation<ref>The C-17 chain of deoxydammarenyl cation is α-configuration, not β as in eukaryotes.<br/> | |
| − | + | ''Ref.'' Wendt KU, Schulz GE, Corey EJ, Liu DR (2000) Enzyme mechanisms for polycyclic triterpene formation. [http://www3.interscience.wiley.com/journal/72515653/abstract?CRETRY=1&SRETRY=0 ''Angew Chem, Int Ed'' 39:2812-33]</ref> <br/>[[Image:Deoxydammarenyl cation.png|150px]] | |
| − | | [[Image: | + | |
| − | + | ||
| − | + | ||
| − | |- | + | |
| − | + | ||
| − | + | ||
| − | | | + | |
|- | |- | ||
| + | | | ||
| + | | | ||
| + | |align="center"| | ||
| + | {| | ||
| + | | D-ring<br/>expansion | ||
| [[Image:Arrow00d35.png]] | | [[Image:Arrow00d35.png]] | ||
| + | | E-ring cyclization<br/> from 17α | ||
| + | |} | ||
|- | |- | ||
| − | | | + | |style="background-color:#ddf"| hopene<br/>[[Image:Hopene.png|150px]] |
| − | + | | [[Image:Arrow00l.png]] | |
| − | | [[Image: | + | | hopyl cation (c-c-c-c)<br/>[[Image:Hopyl cation.png|150px]] |
|} | |} | ||
| − | |||
{{Twocolumn| | {{Twocolumn| | ||
| − | + | Hopanoids are widespread: they are found in bacteria, ferns, and geological sediments. They are not found, however, in archaea or animals. | |
| + | |||
| | | | ||
| + | ホパノイドはバクテリアだけでなく、シダ類や地下堆積物からも見つかっています。しかしアーキアや動物からは見つかっていません。 | ||
| + | }} | ||
| + | {{Twocolumn| | ||
| + | Almost all mono-, di-, tri- and tetracyclic terpenes from squalene are found in ferns: [[Species:Polypodium|Polypodium]], [[Species:Lemmaphyllum|Lemmaphyllum]], and [[Species:Pyrrosia|Pyrrosia]]. Notable exception is ''Z''-Dammara-17(20),24-diene from moss [[Species:Floribundaria|Floribundaria]]<ref>Toyota M, Masuda K, Asakawa Y (1998) Triterpenoid constituents of the moss Floribundaria aurea subsp. nipponica. Phytochemistry 48:297–299</ref>. | ||
| + | Pentacyclic terpenes from squalene, on the other hand, are found also in angiosperms such as [[Species:Achillea|Achillea]], [[Species:Erysimum|Erysimum]], [[Species:Castanopsis|Castanopsis]] and in Ascomycota. | ||
| + | | | ||
| + | スクアレンから生成されるほぼ全ての1,2,3,4環テルペンはシダ類で見つかっています([[Species:Polypodium|Polypodium]], [[Species:Lemmaphyllum|Lemmaphyllum]], and [[Species:Pyrrosia|Pyrrosia]])。例外はコケ類[[Species:Floribundaria|Floribundaria]]からの''Z''-Dammara-17(20),24-diene ぐらいです。 | ||
| + | それに対して、スクアレン由来の5環のテルペンは被子植物([[Species:Achillea|Achillea]], [[Species:Erysimum|Erysimum]], [[Species:Castanopsis|Castanopsis]])や子嚢菌類にも見つかっています。 | ||
}} | }} | ||
| − | == | + | <references/> |
| + | |||
| + | ==={{Bilingual|ビスオキシドスクアレン環化酵素|Bis-oxidosqualene Cyclase}}=== | ||
{{Twocolumn| | {{Twocolumn| | ||
| − | + | Squalene is oxidized by squalene oxidase to become 2,3-oxidosqualene. | |
| + | Further epoxidation of this symmetric molecule produces 2,3-(''S'')-22,23-(''S'')-bis-oxidosqualene, which is converted to 24,25-epoxylanostan-3-ol or 24,25-epoxycycloartan-3-ol. | ||
| + | Epoxylanosterol is known to negatively regulate sterol biosynthesis<ref>Gardner RG, Shan H, Matsuda SPT, Hampton RY (2001) A positive oxysterol-derived signal for 3-hydroxy-3-methylglutaryl CoA reductase degradation in yeast. ''J Biol Chem'' 276:8681–869</ref>. | ||
| | | | ||
| + | スクアレンはスクアレン酸化酵素により2,3-オキシドスクアレンになりますが、対称な分子のため酸化が進むと 2,3-(''S'')-22,23-(''S'')-ビスオキシドスクアレンになります。これからエポキシ末端をもつラノステロールやシクロアルテノールが生じます。 | ||
| + | エポキシラノステロールはHMG-CoA還元酵素の分解を促進してステロール合成の調節をつかさどっていることが酵母で知られています。 | ||
}} | }} | ||
| − | {| style="text-align:center | + | <center> |
| + | {| style="text-align:center" | ||
| + | | [[Image:2,3-oxidosqualene.png]] | ||
| + | | [[Image:2,3-22,23-bis-oxidosqualene.png]] | ||
|- | |- | ||
| − | | | + | | 2,3-oxidosqualene |
| − | + | | 2,3-22,23-bis-oxidosqualene | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
|} | |} | ||
| + | </center> | ||
| + | |||
| + | <references/> | ||
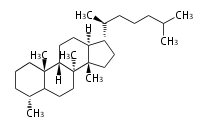
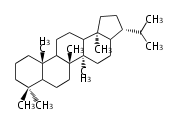
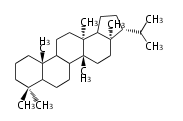
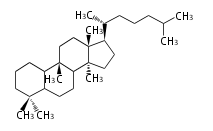
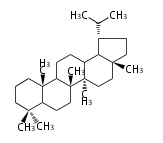
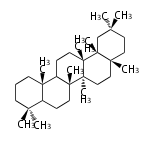
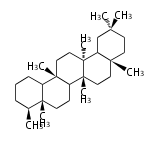
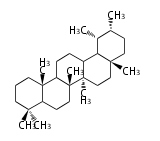
| − | ==Design of Tri-terpene ID numbers | + | =={{Bilingual|ID番号の設計|Design of Tri-terpene ID numbers}}== |
<center> | <center> | ||
12-DIGIT | 12-DIGIT | ||
| Line 281: | Line 388: | ||
; ''y'' ... backbone structure (母核構造) : | ; ''y'' ... backbone structure (母核構造) : | ||
| − | + | ||
{| class="wikitable sortable" | {| class="wikitable sortable" | ||
| − | ! Symbol at ''y'' || Carbons || Steroids | + | ! Symbol at ''y'' || Carbons || Steroids || Backbone Structure |
{{#repeat:TP/Backbone/TableRow|3| | {{#repeat:TP/Backbone/TableRow|3| | ||
GN | GN | ||
| Line 316: | Line 423: | ||
poriferastane | poriferastane | ||
}} | }} | ||
| − | |} | + | |} |
| + | |||
{| class="wikitable sortable" | {| class="wikitable sortable" | ||
| − | ! Symbol at ''y'' || Carbons || | + | ! Symbol at ''y'' || Carbons || Triterpenoids || Backbone Structure |
{{#repeat:TP/Backbone/TableRow|3| | {{#repeat:TP/Backbone/TableRow|3| | ||
| + | PS | ||
| + | C30 | ||
| + | protostane | ||
| + | EU | ||
| + | C30 | ||
| + | euphane | ||
LN | LN | ||
| − | C30 | + | C30 |
lanostane | lanostane | ||
CY | CY | ||
| − | C30 | + | C30 |
cycloartane | cycloartane | ||
| − | + | FS | |
| − | C30 ( | + | C29 |
| − | + | fusidane | |
| + | HP | ||
| + | C30 (5 rings) | ||
| + | hopane | ||
| + | FN | ||
| + | C30 (5 rings) | ||
| + | fernane | ||
CC | CC | ||
| − | C30 | + | C30 |
cucurbitane | cucurbitane | ||
DM | DM | ||
| − | C30 | + | C30 |
dammarane | dammarane | ||
| + | BC | ||
| + | C30 | ||
| + | baccharane | ||
HL | HL | ||
| − | C30 | + | C30 |
| − | holostane | + | holostane |
PF | PF | ||
C29 (5 rings) | C29 (5 rings) | ||
pfaffane | pfaffane | ||
| − | |||
| − | |||
| − | |||
LP | LP | ||
C30 (5 rings) | C30 (5 rings) | ||
| Line 350: | Line 470: | ||
C30 (5 rings) | C30 (5 rings) | ||
oleanane | oleanane | ||
| − | + | FD | |
C30 (5 rings) | C30 (5 rings) | ||
| − | + | friedelane | |
TR | TR | ||
C30 (5 rings) | C30 (5 rings) | ||
| Line 359: | Line 479: | ||
C30 (5 rings) | C30 (5 rings) | ||
ursane | ursane | ||
| + | SR | ||
| + | C30 (5 rings) | ||
| + | serratane | ||
}} | }} | ||
|} | |} | ||
| − | + | ||
; ''r'' ... number of major rings (環構造数) : | ; ''r'' ... number of major rings (環構造数) : | ||
Latest revision as of 11:55, 8 December 2012
Contents |
[edit] Triterpene (C30)
[edit] Ring configuration
[edit] Steroid
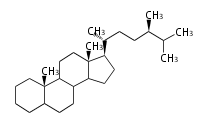
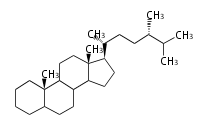
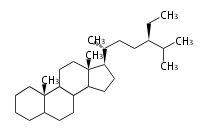
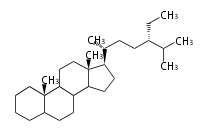
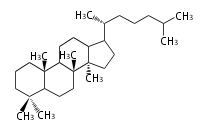
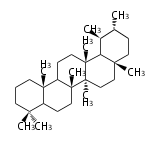
The basic structure is 4 carbon rings, cyclopenta[a]phenanthrene, gonane, or sterane. The rings B/C are always trans in all natural steroids. If the rings C/D are trans, it is called gonane. If its stereochemistry is unspecified, it is called sterane. Most steroids take gonane form, but in cardenolides and bufanolides, the rings C/D are cis.
 |
|
| Cyclopenta[a]phenanthrene | Gonane |
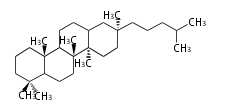
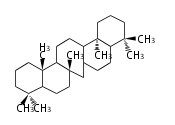
The majority of steroids have methyl groups sticking out from the bridgehead positions C-10 and C-13. When these methyl groups (or hydrogens) stand above the plane, they are called β-configuration. Those below the plane are called α-configuration. If the configuration at any site is unknown, it is indicated as ξ (Greek Xi). By default, hydrogen atoms or substituents at the positions C-8, 9, 10, 13, and 14 are assumed to be 8β, 9α, 10β, 13β, and 14α configurations. C-5 is a special position, because there are as many 5α steroids as 5β are.
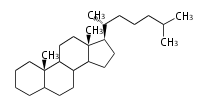 |
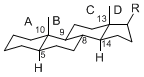 |
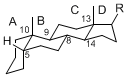
|
| cholestane backbone | 5α-configuration | 5β-configuration |
[edit] Triterpenes
In almost all pentacyclic triterpenes in angiosperms, the methyl group at the DE-ring fusion is β-configuration. Some triterpenes in ferns, mosses, gymnosperms have α-methyl group at the DE-ring fusion.
[edit] Biosynthesis
[edit] Overview
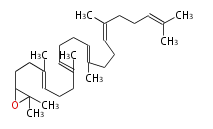
The starting point is squalene, which is formed by joining two FPPs tail-to-tail. Bacterial cyclases use squalene directly[1], but those of the other species use 2,3-oxidosqualene for cyclization.
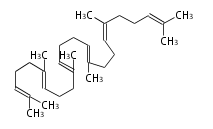
|
|
| squalene | 2,3-oxidosqualene |
- In bacteria, squalene is cyclized via the 17α-deoxydammarenyl cation to hopene and other triterpenes.
- In eukaryotes, 2,3-oxidosqualene is cyclized via the protosteryl cation to lanosterol (animals and fungi), cycloartenol (plants) or parkeol (sea cucumbers) by a series of 1,2-hydride and methyl shifts (Wagner-Meerwein shifts).
- In plants, a trace amount of phytosterols comes from lanosterol [2] At3g45130 is lanosterol synthase in Arabidopsis and its orthologs exist in asterids Taraxacum officinale and Panax ginseng and eurosid Luffa cylindrica. Lanosterol synthase exists broadly among eudicots [3]. Parkeol is also widespread in plants.
- In plants, various triterpenes arise from the 17β-dammarenyl cation.
- References
- ↑ Bacterial squalene cyclases can accept oxidosqualene as their substrates, but oxidosqualene usually does not exist in bacteria
- ↑ Ohyama K, Suzuki M, Kikuchi J, Saito K, Muranaka T (2009) Dual biosynthetic pathways to phytosterol via cycloartenol and lanosterol in Arabidopsis Proc Natl Acad Sci USA 106(3):725-730
- ↑ Kolesnikova MD, Xiong Q, Lodeiro S, Hua L, Matsuda SPT (2006) Lanosterol biosynthesis in plants Arch Biochem Biophys 447:87-95
[edit] Oxidosqualene Cyclase in Eukaryotes
Any path of reactions from the root (2,3-oxidosqualene) to any triterpene backbone with a colored background is catalyzed by a single enzyme called oxidosqualene cyclase (OSC) or terpene synthase h (tpsh).[1]
| Backbone Color Code: | Animals, fungi, and yeast | Plants only | |
|---|---|---|---|
| Six-membered rings: | chair (C), or boat (B) | ||
| 2,3-oxidosqualene | |||||||||||||||||

| |||||||||||||||||
|
| ||||||||||||||||
17β-protosteryl cation (C-B-C)[5] 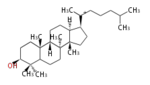
|
1,2-shift |
lanosteryl cation (C-B-C)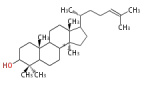
|
17β-dammarenyl cation (C-C-C)[6] 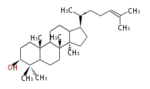
| ||||||||||||||
|
|
| |||||||||||||||
cation with the chain at C18 or C17 position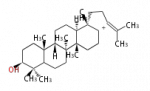 or or 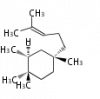
|
|
|
baccarenyl cation (C-C-C-C)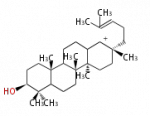
| ||||||||||||||
|
|
|
| ||||||||||||||
arborinyl cation (C-B-C-C)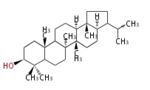
|
unnamed cation (C-B-C-C)
|
21α-hopyl cation (C-C-C-C) 21β-moretyl cation (C-C-C-C)[10] 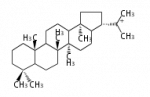
|
H18α-lupyl cation (C-C-C-C) H18β-lupyl cation (C-C-C-B) 
| ||||||||||||||
|
|
|
| ||||||||||||||
| arborinane (C-B-C-C) stictane (C-B-C-C-C)[11] |
hancokinane (C-B-C-C) | hopane (C-C-C-C) gammacerane (C-C-C-C-C) fernane (C-C-C-C) swertane (C-C-C-C-C) |
oleanane[12] (C-C-C-C-C) lupane (C-C-C-C) germanicane taraxastane (C-C-C-C-C) ursane (C-C-C-C-C/B) friedomadeirane (C-C-C-C)[13] | ||||||||||||||
| References |
|---|
|
| Reviews |
|
[edit] Squalene Cyclase in Bacteria and Ferns
Squalene cyclase (SC) or terpene synthase g (tpsg) are found in prokaryotes, ciliates, and lower plants (mosses and ferns) and can convert squalene, which is symmetric, as well as 2,3-oxidosqualene. Main products are hopanol and tetrahymanol, and only generate all-chair cations.
squalene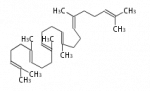
|
squalene-hopene cyclase[1] |
17α-deoxydammarenyl cation[2] 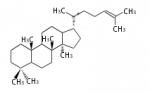
| |||
| |||||
hopene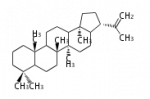
|
|
hopyl cation (c-c-c-c)
|
Hopanoids are widespread: they are found in bacteria, ferns, and geological sediments. They are not found, however, in archaea or animals.
Almost all mono-, di-, tri- and tetracyclic terpenes from squalene are found in ferns: Polypodium, Lemmaphyllum, and Pyrrosia. Notable exception is Z-Dammara-17(20),24-diene from moss Floribundaria[3]. Pentacyclic terpenes from squalene, on the other hand, are found also in angiosperms such as Achillea, Erysimum, Castanopsis and in Ascomycota.
- ↑ SH cyclase is the most investigated enzyme among squalene cyclases.
Ref. Kannenberg EL, Poralla K (1999) Hopanoid biosyntehsis and function in bacteria. Naturwissenschaften 86:168-76. - ↑ The C-17 chain of deoxydammarenyl cation is α-configuration, not β as in eukaryotes.
Ref. Wendt KU, Schulz GE, Corey EJ, Liu DR (2000) Enzyme mechanisms for polycyclic triterpene formation. Angew Chem, Int Ed 39:2812-33 - ↑ Toyota M, Masuda K, Asakawa Y (1998) Triterpenoid constituents of the moss Floribundaria aurea subsp. nipponica. Phytochemistry 48:297–299
[edit] Bis-oxidosqualene Cyclase
Squalene is oxidized by squalene oxidase to become 2,3-oxidosqualene. Further epoxidation of this symmetric molecule produces 2,3-(S)-22,23-(S)-bis-oxidosqualene, which is converted to 24,25-epoxylanostan-3-ol or 24,25-epoxycycloartan-3-ol. Epoxylanosterol is known to negatively regulate sterol biosynthesis[1].

|

|
| 2,3-oxidosqualene | 2,3-22,23-bis-oxidosqualene |
- ↑ Gardner RG, Shan H, Matsuda SPT, Hampton RY (2001) A positive oxysterol-derived signal for 3-hydroxy-3-methylglutaryl CoA reductase degradation in yeast. J Biol Chem 276:8681–869
[edit] Design of Tri-terpene ID numbers
12-DIGIT
| T | P | 3 | x | y | y | r | h | g | n | c | c |
- x ... species information
| Symbol at x | Kingdom | Phyla | Examples |
|---|---|---|---|
| I | Animalia | Arthropoda (Insects, crabs) | ecdysteroids |
| V | Chordate (Vertebrates) | sex steroids, corticosteroids, anabolic steroids | |
| O | Others | marine steroids | |
| P | Plantae | Phytosterols | lanosterols, cholesterols, brassinolides |
| S | Saponins | saponins | |
| F | Fungi | ergosterols | ergosterols |
| B | Bacteria | bacterial sterols | hopanoids |
- y ... backbone structure (母核構造)
- r ... number of major rings (環構造数)
Click above categories to see details.
- h ... hydroxylation pattern (水酸基数)
Click above categories to see details.
- g ... glycosylation pattern(糖修飾パターン)
Click above categories to see details.
- n ... number of sugars (修飾糖数)
Click above categories to see details.
- c ... serial number (通し番号)
This category currently contains no pages or media.