Category:FLI
m |
m (→{{Bilingual|生合成|Biosynthesis}}) |
||
| (17 intermediate revisions by one user not shown) | |||
| Line 3: | Line 3: | ||
{{Hierarchy|{{PAGENAME}}}} | {{Hierarchy|{{PAGENAME}}}} | ||
| + | =={{Bilingual|イソフラボノイドの概要|Overview}}== | ||
{| class="wikitable" border="1" cellspacing="0" | {| class="wikitable" border="1" cellspacing="0" | ||
|- | |- | ||
| Line 20: | Line 21: | ||
|FLIJ||[[:Category:FLIJ|alpha-Methoxynbenzoin]]<br>[[Image:Flij.png|90px]] | |FLIJ||[[:Category:FLIJ|alpha-Methoxynbenzoin]]<br>[[Image:Flij.png|90px]] | ||
|} | |} | ||
| − | |||
| − | |||
{{Twocolumn| | {{Twocolumn| | ||
| − | The discovery of isoflavonoid was in 1842, when Reinsch and Hlasiwetz obtained ononin from the roots of spiny restharrow ([[Species:Ononis|''Ononis spinosa'' L.]]). Its structure was later determined by [[Reference:Baker_W:Robinson_R:Simpson_NM:%2CJ._Chem._Soc.%2C1933%2C_%2C274|Baker et al.]] as [[FLIA1AGS0002|Formononetin 7-O-glucoside]] (1933). Since then, most isoflavonoids have been found in [[:Category:Fabaceae|Fabaceae]] (bean family).</ | + | The discovery of isoflavonoid was in 1842, when Reinsch and Hlasiwetz obtained ononin from the roots of spiny restharrow ([[Species:Ononis|''Ononis spinosa'' L.]])<ref>Reinsch H, ''Repert. Pharm.'' 26:12-31, 1842; Reinsch H, ''Repert. Pharm.'' 28:18-25, 1842; Hlasiwetz H, ''J. Prakt. Chem.'' 65:419-450, 1855; Hlasiwetz H, ''Sitzungsber. Kais. Akad. Wiss. Wien, Math.-Naturwiss. Kl.'' 15:142-168, 1855</ref>. Its structure was later determined by [[Reference:Baker_W:Robinson_R:Simpson_NM:%2CJ._Chem._Soc.%2C1933%2C_%2C274|Baker et al.]] as [[FLIA1AGS0002|Formononetin 7-O-glucoside]] (1933). Since then, most isoflavonoids have been found in [[:Category:Fabaceae|Fabaceae]] (bean family). |
| − | + | <br/> | |
| − | First isoflavonoid found in non-bean family was iridin from the rhizomes of [[Species:Iris|''Iris florentina'']] ([[:Category:Iridaceae|Iridaceae]]) | + | First isoflavonoid found in non-bean family was [[FLIAEGGS0001|iridin]] from the rhizomes of [[Species:Iris|''Iris florentina'']] ([[:Category:Iridaceae|Iridaceae]])<ref>de Laire G and Tiemann F, ''Chem. Ber.'', 26:2010, 1893</ref>. The first structural identification was [[FLIACANS0001|Prunetin]] from [[Species:Prunus|Prunus]] species in 1910 by [[Reference:Finnemore_H:%2CPharm._J.%2C1910%2C31%2C604|Finnemore]]. Major isoflavonoids in iris are isoflavones and their O-glycosides. In iris, isoflavonoids are produced only in the rhizomes except for a trace of coumaranochromones in leaves <ref>Iwashita T, Ootani S: Flavonoids of the genus Iris: structures, distribution and function (review). Ann. Tsukuba Bot. Gard. 17:147-183 (in Japanese)</ref>. |
| | | | ||
イソフラボノイドの発見は1842年まで遡り、ReinschとHlasiwetzが | イソフラボノイドの発見は1842年まで遡り、ReinschとHlasiwetzが | ||
[[Species:Ononis|マメ科ハリモクシュ]]にononinを見出しました。 | [[Species:Ononis|マメ科ハリモクシュ]]にononinを見出しました。 | ||
その構造を[[FLIA1AGS0002|Formononetin 7-O-glucoside]]と同定したのは [[Reference:Baker_W:Robinson_R:Simpson_NM:%2CJ._Chem._Soc.%2C1933%2C_%2C274|Baker et al.]] たちです(1933)。以降、イソフラボノイドのほとんどは[[:Category:Fabaceae|マメ科]]に見出されてきました。 | その構造を[[FLIA1AGS0002|Formononetin 7-O-glucoside]]と同定したのは [[Reference:Baker_W:Robinson_R:Simpson_NM:%2CJ._Chem._Soc.%2C1933%2C_%2C274|Baker et al.]] たちです(1933)。以降、イソフラボノイドのほとんどは[[:Category:Fabaceae|マメ科]]に見出されてきました。 | ||
| − | </ | + | <br/> |
| − | マメ科以外からはじめて見つかったイソフラボノイドは、アヤメ([[Species:Iris|''Iris florentina'']], [[:Category:Iridaceae|Iridaceae]]) | + | マメ科以外からはじめて見つかったイソフラボノイドは、アヤメ([[Species:Iris|''Iris florentina'']], [[:Category:Iridaceae|Iridaceae]])の根茎から抽出された[[FLIAEGGS0001|イリジン]]です。ただし初めて精製、構造が同定されたのは1910年の[[FLIACANS0001|プルネチン]] ([[Species:Prunus|Prunus]]属) です([[Reference:Finnemore_H:%2CPharm._J.%2C1910%2C31%2C604|Finnemore]])。アヤメにおける主なイソフラボノイドはイソフラボンとその配糖体です。アヤメでは葉に微量あるクマラノクロモンを除く全イソフラボノイドが根茎にあります。 |
}} | }} | ||
| − | |||
| − | |||
| − | |||
| − | + | <references/> | |
| − | + | ||
| − | + | ==={{Bilingual|代表例|Representative Names}}=== | |
| − | + | {| style="vertical-align:top" | |
| − | + | | [[FLIA1ANS0002|daidzein]]<br/>[[Image:FLIA1ANS0002.png|120px]] | |
| − | </ | + | | [[FLIAAANS0001|genistein]]<br/>[[Image:FLIAAANS0001.png|120px]] |
| + | | [[FLIE1ANS0001|coumestrol]]<br/>[[Image:FLIE1ANS0001.png|120px]] | ||
| + | | [[FLID1ANS0001|Medicarpin]]<br/>[[Image:FLID1ANS0001.png|120px]] | ||
| + | |} | ||
| + | |||
| + | {{Twocolumn| | ||
| + | [[FLIA1AGS0001|Daidzin]] is its glycosylated form in soy beans. Daidzein (aglycon) is contained in fermented beans. | ||
| + | Medicarpin is the major phytoalexin in alfalfa ([[Species:Medicago|''Medicago sativa'']]) and clover ([[Species:Trifolium|Trifolium spp.]]), synthesized by bacterial infection<ref>Guo L, Dixon RA, Paiva NL (1994) Conversion of vestitone to medicarpin in alfalfa (Medicago sativa L.) is catalyzed by two independent enzymes. Identification, purification, and characterization of vestitone reductase and 7,2'-dihydroxy-4'-methoxyisoflavanol dehydratase. ''J Biol Chem'' 269:22372-22378</ref>. | ||
| + | | | ||
| + | 大豆に含まれるのは、[[FLIA1AGS0001|ダイジン]]というダイゼインの配糖体。豆を醗酵させるとアグリコンであるダイゼインになる。 | ||
| + | メディカルパンはアルファルファ([[Species:Medicago|''Medicago sativa'']])やクローバー([[Species:Trifolium|Trifolium spp.]])が微生物の感染に対抗して生産する防御物質として知られる。 | ||
| + | }} | ||
| + | <references/> | ||
| + | |||
| + | =={{Bilingual|食品含有量|Food contents}}== | ||
| + | |||
| + | {| class="wikitable" | ||
| + | ! colspan="5"| Isoflavone Contents (mg / 100g wet weight) <ref>荒井綜一 他編 「機能性食品の辞典」朝倉書店 p.341</ref> | ||
| + | |- | ||
| + | ! food || daizein || genistein || glycitein || total isoflavone | ||
| + | |- | ||
| + | | {{Bilingual|大豆(全粒)乾|soybean (whole, dry)}} || 153 || 201 || 40 || 394 | ||
| + | |- | ||
| + | | {{Bilingual|大豆(全粒)ゆで|soybean (whole, boiled)}} || 27 || 95 || 1 || 123 | ||
| + | |- | ||
| + | | {{Bilingual|えだまめ 生|green soybean (whole, raw)}} || 3 || 6 || 0 || 10 | ||
| + | |- | ||
| + | | {{Bilingual|えだまめ ゆで|green soybean (whole, boiled)}} || 5 || 7 || 1 || 12 | ||
| + | |- | ||
| + | | {{Bilingual|豆腐(木綿、絹、ゆし等)|tofu}} || 12-28 || 20-40 || 2 || 39-63 | ||
| + | |- | ||
| + | | {{Bilingual|納豆、生揚げ、がんもどき|natto, fried tofu}} || 13-37 || 27-82 || 1-4 || 42-126 | ||
| + | |- | ||
| + | | {{Bilingual|味噌(甘味噌、淡色辛、赤色辛)|bean pastes}} || 21-23 || 29-38 || 2-4 || 53-60 | ||
| + | |- | ||
| + | | {{Bilingual|緑豆もやし、アルファルファ、えんどう、いんげん、そらまめ|other beans}} || 0 || 0 || 0 || 0 | ||
| + | |} | ||
| + | <references/> | ||
| − | ==Biosynthesis== | + | =={{Bilingual|生合成|Biosynthesis}}== |
{{Twocolumn| | {{Twocolumn| | ||
This pathway is said to exist as an enzyme complex at ER membrane. <ref>Hrazdina G: Compartmentation in aromatic metabolism. In HA Stafford, RK Ibrahim, eds, Recent Advances in Phytochemistry. Plenum Press, New York, 1992 pp 1–23</ref> | This pathway is said to exist as an enzyme complex at ER membrane. <ref>Hrazdina G: Compartmentation in aromatic metabolism. In HA Stafford, RK Ibrahim, eds, Recent Advances in Phytochemistry. Plenum Press, New York, 1992 pp 1–23</ref> | ||
| | | | ||
| − | + | この経路はER膜に局在する酵素複合体を形成すると言われています。 | |
}} | }} | ||
| + | |||
| + | {| style="text-align:center" | ||
| + | |- | ||
| + | | style="background:whitesmoke"| liquiritigenin<br/>[[Image:FL2F1ANS0001.png|70px]] | ||
| + | | <br/>[[Image:Arrow00r35.png]]<br/>IFS | ||
| + | | style="background:oldlace" | daidzein<br/>[[Image:FLIA1ANS0002.png|70px]] | ||
| + | | <br/>[[Image:Arrow00r35.png]]<br/>IOMT | ||
| + | | style="background:oldlace" | formononetin<br/>[[Image:FLIA1ANS0001.png|70px]] | ||
| + | | <br/>[[Image:Arrow00r35.png]]<br/>I2'H + IFR | ||
| + | | style="background:whitesmoke" | vestitone<br/>[[Image:FLIB1LNS0002.png|70px]] | ||
| + | | <br/>[[Image:Arrow00r35.png]]<br/>VR + DMID | ||
| + | | style="background:mistyrose" | medicarpin<br/>[[Image:FLID1ANS0001.png|70px]] | ||
| + | |} | ||
| + | |||
{| class="wikitable collapsible" | {| class="wikitable collapsible" | ||
| Line 60: | Line 107: | ||
| IFS | | IFS | ||
| isoflavone synthase | | isoflavone synthase | ||
| − | | cytochrome P450 oxygenase family <ref>Akashi T, Aoki T, Ayabe S: Cloning and functional expression of a cytochrome P450 cDNA encoding 2-hydroxyisoflavanone synthase involved in biosynthesis of the isoflavonoid skeleton in licorice. Plant Physiol 1999 121:821-828</ref> | + | | cytochrome P450 oxygenase family <ref>Akashi T, Aoki T, Ayabe S: Cloning and functional expression of a cytochrome P450 cDNA encoding 2-hydroxyisoflavanone synthase involved in biosynthesis of the isoflavonoid skeleton in licorice. Plant Physiol 1999 121:821-828</ref><ref>Steele CL, Gijzen M, Qutob D, Dixon RA: Molecular characterization of the enzyme catalyzing the aryl migration reaction of isoflavonoid biosynthesis in soybean. Arch Biochem Biophys 1999 367: 146–150</ref> |
| − | <ref>Steele CL, Gijzen M, Qutob D, Dixon RA: Molecular characterization of the enzyme catalyzing the aryl migration reaction of isoflavonoid biosynthesis in soybean. Arch Biochem Biophys 1999 367: 146–150</ref> | + | |
<ref>Jung W, Yu O, Sze-Mei CL, O’Keefe DP, Odell J, Fader G, McGonigle B: Identification and expression of isoflavone synthase, the key enzyme for biosynthesis of isoflavones in legumes. Nat Biotechnol 2000 18: 208–213</ref> | <ref>Jung W, Yu O, Sze-Mei CL, O’Keefe DP, Odell J, Fader G, McGonigle B: Identification and expression of isoflavone synthase, the key enzyme for biosynthesis of isoflavones in legumes. Nat Biotechnol 2000 18: 208–213</ref> | ||
| + | |||
| + | * CYP93C subfamily in Fabaceae<ref>Ayabe S, Akashi T (2006) ''Phytochem Rev'' 5(2-3), 271-282</ref> | ||
| + | * ''IgIFS1'' in German iris (Iridaceae) shows low similarity (< 35%) with other P450<ref>Akashi T (2011) Proceedings of Biosynthetic Machinary (in Japanese)</ref> | ||
| Use both liquiritigenin and naringenin as substrates to produce genistein and daidzein, respectively. | | Use both liquiritigenin and naringenin as substrates to produce genistein and daidzein, respectively. | ||
|- | |- | ||
| Line 88: | Line 137: | ||
<references/> | <references/> | ||
| − | ==Database statistics | + | |
| + | =={{Bilingual|データベース統計|Database statistics}}==<!--- see FL1---> | ||
{{#def:FLI|{{#SearchTitle:FLI|}}}} | {{#def:FLI|{{#SearchTitle:FLI|}}}} | ||
{{#def:rFLI|{{#SearchLine:&&FLI|Reference}}}} | {{#def:rFLI|{{#SearchLine:&&FLI|Reference}}}} | ||
| Line 117: | Line 167: | ||
}} | }} | ||
| + | {{FL_pie_chart|FLI}} | ||
[[Category:FL]] | [[Category:FL]] | ||
Latest revision as of 16:26, 4 June 2011
Isoflavonoid
| Flavonoid Top | Molecule Index | Author Index | Journals | Structure Search | Food | New Input |
Upper classes : FL Flavonoid
Contents |
[edit] Overview
| 2nd Class | |||||||
|---|---|---|---|---|---|---|---|
| FLIA | Isoflavone
|
FLIB | Isoflavanone
|
FLIC | Isoflavan
|
FLID | Pterocarpane
|
| FLIE | Coumestan
|
FLIF | Rotenoid
|
FLIG | Coumaranochromone
|
FLIH | 3-Arylcoumarin
|
| FLII | 2-Arylbenzofuran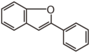
|
FLIJ | alpha-Methoxynbenzoin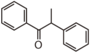
| ||||
The discovery of isoflavonoid was in 1842, when Reinsch and Hlasiwetz obtained ononin from the roots of spiny restharrow (Ononis spinosa L.)[1]. Its structure was later determined by Baker et al. as Formononetin 7-O-glucoside (1933). Since then, most isoflavonoids have been found in Fabaceae (bean family).
First isoflavonoid found in non-bean family was iridin from the rhizomes of Iris florentina (Iridaceae)[2]. The first structural identification was Prunetin from Prunus species in 1910 by Finnemore. Major isoflavonoids in iris are isoflavones and their O-glycosides. In iris, isoflavonoids are produced only in the rhizomes except for a trace of coumaranochromones in leaves [3].
- ↑ Reinsch H, Repert. Pharm. 26:12-31, 1842; Reinsch H, Repert. Pharm. 28:18-25, 1842; Hlasiwetz H, J. Prakt. Chem. 65:419-450, 1855; Hlasiwetz H, Sitzungsber. Kais. Akad. Wiss. Wien, Math.-Naturwiss. Kl. 15:142-168, 1855
- ↑ de Laire G and Tiemann F, Chem. Ber., 26:2010, 1893
- ↑ Iwashita T, Ootani S: Flavonoids of the genus Iris: structures, distribution and function (review). Ann. Tsukuba Bot. Gard. 17:147-183 (in Japanese)
[edit] Representative Names
daidzein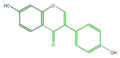
|
genistein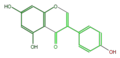
|
coumestrol
|
Medicarpin
|
Daidzin is its glycosylated form in soy beans. Daidzein (aglycon) is contained in fermented beans. Medicarpin is the major phytoalexin in alfalfa (Medicago sativa) and clover (Trifolium spp.), synthesized by bacterial infection[1].
- ↑ Guo L, Dixon RA, Paiva NL (1994) Conversion of vestitone to medicarpin in alfalfa (Medicago sativa L.) is catalyzed by two independent enzymes. Identification, purification, and characterization of vestitone reductase and 7,2'-dihydroxy-4'-methoxyisoflavanol dehydratase. J Biol Chem 269:22372-22378
[edit] Food contents
| Isoflavone Contents (mg / 100g wet weight) [1] | ||||
|---|---|---|---|---|
| food | daizein | genistein | glycitein | total isoflavone |
| soybean (whole, dry) | 153 | 201 | 40 | 394 |
| soybean (whole, boiled) | 27 | 95 | 1 | 123 |
| green soybean (whole, raw) | 3 | 6 | 0 | 10 |
| green soybean (whole, boiled) | 5 | 7 | 1 | 12 |
| tofu | 12-28 | 20-40 | 2 | 39-63 |
| natto, fried tofu | 13-37 | 27-82 | 1-4 | 42-126 |
| bean pastes | 21-23 | 29-38 | 2-4 | 53-60 |
| other beans | 0 | 0 | 0 | 0 |
- ↑ 荒井綜一 他編 「機能性食品の辞典」朝倉書店 p.341
[edit] Biosynthesis
This pathway is said to exist as an enzyme complex at ER membrane. [1]
| liquiritigenin |
IFS |
daidzein |
IOMT |
formononetin |
I2'H + IFR |
vestitone |
VR + DMID |
medicarpin |
| Structural Genes (continued from Flavonoid) | |||
|---|---|---|---|
| Abbrev. | Name | Origin | Information |
| IFS | isoflavone synthase | cytochrome P450 oxygenase family [2][3] | Use both liquiritigenin and naringenin as substrates to produce genistein and daidzein, respectively. |
| IOMT | isoflavone O-methyltransferase | ||
| I2'H | isoflavone 2'-hydroxylase | cytochrome P450 oxygenase family[7] | |
| IFR | isoflavone reductase | ||
| VR | vestitone reductase | ||
| DMID | 7,2'-dihydroxy,4'-methyxyisoflavanol dehydratase | ||
- ↑ Hrazdina G: Compartmentation in aromatic metabolism. In HA Stafford, RK Ibrahim, eds, Recent Advances in Phytochemistry. Plenum Press, New York, 1992 pp 1–23
- ↑ Akashi T, Aoki T, Ayabe S: Cloning and functional expression of a cytochrome P450 cDNA encoding 2-hydroxyisoflavanone synthase involved in biosynthesis of the isoflavonoid skeleton in licorice. Plant Physiol 1999 121:821-828
- ↑ Steele CL, Gijzen M, Qutob D, Dixon RA: Molecular characterization of the enzyme catalyzing the aryl migration reaction of isoflavonoid biosynthesis in soybean. Arch Biochem Biophys 1999 367: 146–150
- ↑ Jung W, Yu O, Sze-Mei CL, O’Keefe DP, Odell J, Fader G, McGonigle B: Identification and expression of isoflavone synthase, the key enzyme for biosynthesis of isoflavones in legumes. Nat Biotechnol 2000 18: 208–213
- ↑ Ayabe S, Akashi T (2006) Phytochem Rev 5(2-3), 271-282
- ↑ Akashi T (2011) Proceedings of Biosynthetic Machinary (in Japanese)
- ↑ Akashi T, Aoki T, Ayabe S: CYP81E1, a cytochrome P450 cDNA of licorice (Glycyrrhiza echinata L.), encodes isoflavone 29-hydroxylase. 1998 Biochem Biophys Res Comm 251: 67–70
[edit] Database statistics
[edit] Major Plant Families
|
|
The number in each family is counted as the number of genera (not species) listed in our registered references. Each reference record is accessible by clicking the link in compound pages. The taxonomy follows the APG-II classification. For details (or if the figure is broken), visit this page. 各科のカウントは種名でなく文献に記載された属名の数です。文献は代謝物ページのリンクからたどれ、分類はAPG-IIです。左の図が表示されない場合はここをクリックしてください。 |