Category:TP3
m (→Design of Tri-terpene ID numbers ID番号の設計) |
(→Design of Tri-terpene ID numbers ID番号の設計) |
||
| Line 153: | Line 153: | ||
! Symbol at ''y'' || Carbons || Triterpenoids || Backbone Structure | ! Symbol at ''y'' || Carbons || Triterpenoids || Backbone Structure | ||
{{#repeat:TP/Backbone/TableRow|3| | {{#repeat:TP/Backbone/TableRow|3| | ||
| + | PS | ||
| + | C30 | ||
| + | protostane | ||
LN | LN | ||
| − | C30 | + | C30 |
lanostane | lanostane | ||
CY | CY | ||
| − | C30 | + | C30 |
cycloartane | cycloartane | ||
| − | + | FS | |
| − | C30 ( | + | C29 |
| − | + | fusidane | |
| + | HP | ||
| + | C30 (5 rings) | ||
| + | hopane | ||
CC | CC | ||
| − | C30 | + | C30 |
cucurbitane | cucurbitane | ||
DM | DM | ||
| − | C30 | + | C30 |
dammarane | dammarane | ||
| + | BC | ||
| + | C30 | ||
| + | baccharane | ||
HL | HL | ||
| − | C30 | + | C30 |
holostane | holostane | ||
PF | PF | ||
C29 (5 rings) | C29 (5 rings) | ||
pfaffane | pfaffane | ||
| − | |||
| − | |||
| − | |||
LP | LP | ||
C30 (5 rings) | C30 (5 rings) | ||
| Line 183: | Line 189: | ||
C30 (5 rings) | C30 (5 rings) | ||
oleanane | oleanane | ||
| − | |||
| − | |||
| − | |||
TR | TR | ||
C30 (5 rings) | C30 (5 rings) | ||
| Line 192: | Line 195: | ||
C30 (5 rings) | C30 (5 rings) | ||
ursane | ursane | ||
| + | SR | ||
| + | C30 (5 rings) | ||
| + | serratane | ||
}} | }} | ||
|} | |} | ||
Revision as of 13:39, 4 August 2010
Contents |
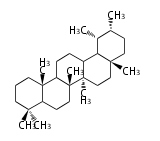
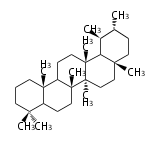
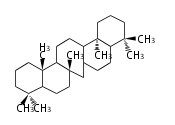
Triterpene (C30) Classes
Biosynthesis
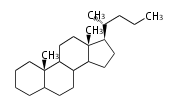
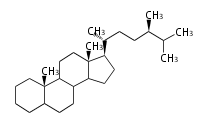
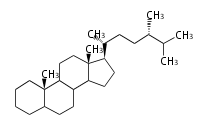
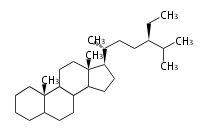
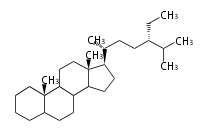
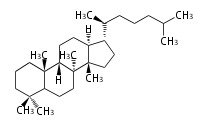
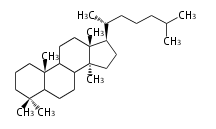
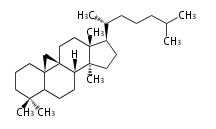
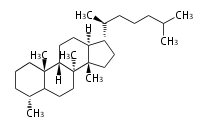
Triterpenes are formed by joining two FPPs tail-to-tail. The precursor compound of cholesterol (C27) is lanosterol (C30) for animals. For plants, fungi and algae, it is almost cycloartenol with a trace of lanosterol-derived sterols[1].
Ring configuration
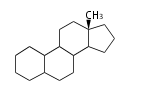
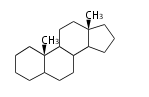
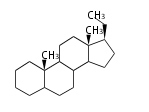
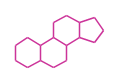
The basic structure is 4 carbon rings, cyclopenta[a]phenanthrene, gonane, or sterane. The rings B/C are always trans in all natural steroids. If the rings C/D are trans, it is called gonane. If its stereochemistry is unspecified, it is called sterane. Most steroids take gonane form, but in cardenolides and bufanolides, the rings C/D are cis.
 |
|
| Cyclopenta[a]phenanthrene | Gonane |
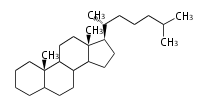
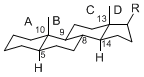
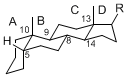
The majority of steroids have methyl groups sticking out from the bridgehead positions C-10 and C-13. When these methyl groups (or hydrogens) stand above the plane, they are called β-configuration. Those below the plane are called α-configuration. If the configuration at any site is unknown, it is indicated as ξ (Greek Xi). By default, hydrogen atoms or substituents at the positions C-8, 9, 10, 13, and 14 are assumed to be 8β, 9α, 10β, 13β, and 14α configurations. C-5 is a special position, because there are as many 5α steroids as 5β are.
 |
 |
|
| cholestane backbone | 5α-configuration | 5β-configuration |
Design of Tri-terpene ID numbers ID番号の設計
12-DIGIT
| T | P | 3 | x | y | y | r | h | g | n | c | c |
- x ... species information
| Symbol at x | Kingdom | Phyla | Examples |
|---|---|---|---|
| I | Animalia | Arthropoda (Insects, crabs) | ecdysteroids |
| V | Chordate (Vertebrates) | sex steroids, corticosteroids, anabolic steroids | |
| O | Others | marine steroids | |
| P | Plantae | Phytosterols | lanosterols, cholesterols, brassinolides |
| S | Saponins | saponins | |
| F | Fungi | ergosterols | ergosterols |
| B | Bacteria | bacterial sterols | hopanoids |
- y ... backbone structure (母核構造)
- r ... number of major rings (環構造数)
Click above categories to see details.
- h ... hydroxylation pattern (水酸基数)
Click above categories to see details.
- g ... glycosylation pattern(糖修飾パターン)
Click above categories to see details.
- n ... number of sugars (修飾糖数)
Click above categories to see details.
- c ... serial number (通し番号)
Cite error:
<ref> tags exist, but no <references/> tag was found
This category currently contains no pages or media.