Category:TP
m (→Biosynthesis Overview) |
m (→Overview) |
||
| Line 3: | Line 3: | ||
{{Twocolumn| | {{Twocolumn| | ||
Terpenes are present in all living organisms. | Terpenes are present in all living organisms. | ||
| − | The word 'terpene' originates from turpentine, the distillation of resin from pine trees ([[:Category:Pinaceae|Pinaceae]]). Chemically speaking, terpenes or terpenoids refer to all natural compounds build up from isoprene (C5, 2-methyl-1,3-butadiene) units. | + | The word 'terpene' originates from turpentine, the distillation of resin from pine trees ([[:Category:Pinaceae|Pinaceae]]) whose main ingredients are α- and β-pinene. Chemically speaking, terpenes or terpenoids refer to all natural compounds build up from isoprene (C5, 2-methyl-1,3-butadiene) units. |
For this reason, terpenes are also called isoprenoids. | For this reason, terpenes are also called isoprenoids. | ||
| − | |||
| − | |||
| | | | ||
| − | + | テルペンという言葉は松の木の脂を蒸留して得られるテレビン油 (turpentine)に由来しますが (主成分はα- および β-ピネン)、全ての生物中に見出されます。 | |
| − | テルペンという言葉は松の木の脂を蒸留して得られるテレビン油 (turpentine) | + | |
化学的には、C5ユニットのイソプレン (2-メチル 1,3-ブタジエン) から構成される天然化合物を全てテルペンまたはテルペノイドと呼びます。 | 化学的には、C5ユニットのイソプレン (2-メチル 1,3-ブタジエン) から構成される天然化合物を全てテルペンまたはテルペノイドと呼びます。 | ||
この理由で、テルペンのことをイソプレノイドとも呼びます。 | この理由で、テルペンのことをイソプレノイドとも呼びます。 | ||
| − | <br/> | + | }} |
| − | + | <center> | |
| − | + | {| | |
| − | + | | [[Image:isoprene.png]]<br/>isoprene | |
| + | | <big>Terpene = Terpenoid = Isoprenoid</big> | ||
| + | |} | ||
| + | </center> | ||
| + | {{Twocolumn| | ||
| + | Well known terpenes include myrrh from balm trees, fragrance or essential oils of herbs and roses. Biologically, terpenes function as volatile expellant of herbivores and attractant of pollinators, or less volatile bitter antifeedants. Many hormones, pheromones and signal compounds are also derived from terpenes. | ||
| + | | | ||
| + | 身近なテルペンの具体例として、香木から得られるミルラ(没薬)、ハーブやバラ等のエッセンシャルオイルや香り成分が挙げられます。 | ||
| + | 生物学的な機能としては、草食動物の忌避物質、花粉を運ぶ昆虫の誘引物質、苦味成分による摂食阻害物質としても働きます。 | ||
| + | 多くのホルモン、フェロモン、シグナル伝達物質がテルペン由来です。 | ||
}} | }} | ||
Revision as of 11:38, 3 August 2010
Contents |
Terpenes
Overview
Terpenes are present in all living organisms. The word 'terpene' originates from turpentine, the distillation of resin from pine trees (Pinaceae) whose main ingredients are α- and β-pinene. Chemically speaking, terpenes or terpenoids refer to all natural compounds build up from isoprene (C5, 2-methyl-1,3-butadiene) units. For this reason, terpenes are also called isoprenoids.
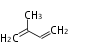 isoprene |
Terpene = Terpenoid = Isoprenoid |
Well known terpenes include myrrh from balm trees, fragrance or essential oils of herbs and roses. Biologically, terpenes function as volatile expellant of herbivores and attractant of pollinators, or less volatile bitter antifeedants. Many hormones, pheromones and signal compounds are also derived from terpenes.
History
- The German chemist Otto Wallach proposed the construction of terpenes from isoprene units known as the isoprene rule[1], and received the Nobel Prize in Chemistry in 1910.
- In 1930s Leopold Ruzicka rationalized the reaction mechanisms of isoprene units and proposed the biogenetic isoprene rule, in which all compounds derived from active isoprene units should be included as terpenes. This active isoprene is now known as isopentenyl di- (or pyro-) phosphate (IPP). He received the Nobel Prize in Chemistry in 1939.[2] More details are explained in the Reference [3]
- In 1999, Rohmer et al. elucidated the non-mevalonate pathway or MEP pathway.
- ↑ Wallach O. (1887) Zur kenntniss der terpene und der ätherischen öle. Justus Liebigs Ann Chem 239:1-54
- ↑ Ruzicka, L (1953) "The isoprene rule and the biogenesis of terpenic compounds" Cell. Mol. Life Sci. 9(10): 357-367
- ↑ Eschenmoser A, Arigoni D (2005) "Revisited after 50 Years: The ‘Stereochemical Interpretation of the Biogenetic Isoprene Rule for the Triterpenes’" Helvetica Chimica Acta 88(12), 3011-50
Classification
Depending on the number of isoprene units used, terpenoids are classified as follows.
- hemi-terpenes (C5)
- mono- (C10)
- sesqui- (C15)
- di- (C20)
- sester- (C25)
- tri- (C30)
- tetra- (C40)
- poly- (C5)n (n > 8)
Biosynthesis
The common precursor of terpenes is isopentenyl diphosphate (IPP) and its isomer, dimethylallyl diphosphate (DMAPP). Both are synthesized through the mevalonate pathway (MVA pathway).
| IPP | DMAPP | |
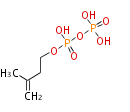
|
IPP isomerase EC5.3.3.2 |
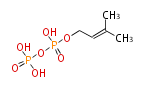
|
Mevalonate (MVA) Pathway
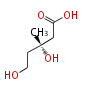
In the MVA pathway, 3 molecules of acetyl CoA are first condensed to produce 3-hydroxy-3-methyl-glutaryl CoA (HMG-CoA), which is then reduced using 2 molecules of NADPH to form 3(R)-mevalonic acid (MVA). The reduction by HMG-CoA reductase is considered the rate-limiting step of IPP biosynthesis (and therefore cholesterol biosynthesis), and the well-known drugs "statin" prescribed for hypercholesterolemia are HMG-CoA reductase inhibitors[1]. Mevalonic acid is then twice phosphorylated by 2 molecules of ATP to form IPP.
| HMG-CoA | (3R)-MVA | IPP | ||
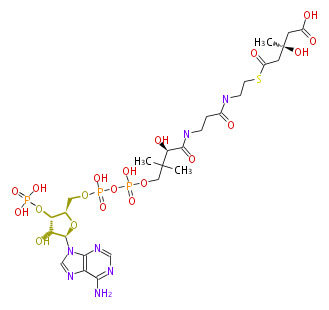
|
HMG-CoA reductase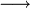 EC1.1.1.34 |
|
kinase & decarboxylase EC2.7.4.2 & EC4.1.1.33 |

|
The plant HMG-CoA reductases are also rate-limiting and are located in the ER membrane. Upon wounding or pathogen infection they are activated to prouduce terpenes.
- ↑ Endo A “The discovery and development of HMG-CoA reductase inhibitors” J Lipid Res 33 (11): 1569–1582, 1992
Methylerythritol-phosphate (MEP) Pathway
In plants, terpenes are synthesized through the methylerythritol phosphate (MEP) pathway.
This MVA-independent pathway is also referred to as deoxyxylulose phosphate (DXP) or , but the name "MEP" is preferred to specify the terpenoid biosynthesis, because DXP is also known as a precursor of pyridoxal, the major form of Vitamin B6 in animal tissues (Vitamin B6 includes pyridoxal, pyridoxamine, and pyridoxine and their phosphate forms) [1].
Acetyl moiety from pyruvic acid is transferred onto D-glyceraldehyde 3-phosphate using a reverse aldol mechanism in an enzyme-bound fashion to form 1-deoxy-D-xylulose 5-phosphate. Then it undergoes a Pinacol rearrangement to form a branched chain compound, 2-C-methyl-D-erythritol 4-phosphate (MEP). MEP is cytidyl-diphosphorylated by CTP and its 2-hydroxyl position is phosphorylated by ATP. Then cyclization occurs, producing 2-C-methyl-D-erythritol-2,4-cyclophosphate. IPP and DMAPP are produced from the cyclic anhydride in still unidentified enzymatic steps using NADPH.
- ↑ Tambasco-Studart M, Titiz O, Raschle T, Forster G, Amrhein N, Fitzpatrick TB “Vitamin B6 biosynthesis in higher plants” Proc Natl Acad Sci USA 102(38):13687-13692, 2005
Biosynthesis Overview
Animals and fungi lack the MEP pathway and exclusively utilize the MVA pathway in cytosol [1]. Bacteria and plants use both pathways but selectively: IPP is turned into farnesyl diphosphate (C15) in the cytosol and the cytosol/endoplasmic reticulum boundary, and the MVA pathway is largely responsible for sesquiterpenoids (C15), steroids (C30), and triterpenoids (C30) [2]. On the other hands, enzymes related to the MEP pathway exist in plastids, and monoterpenoids (C10), diterpenoids (C20) and carotenoids (C40) are synthesized mainly there [3][4].
... reactions in plastids
... reactions in the cytosol & ER
- (h-t) ... head-to-tail conjugation
- (t-t) ... tail-to-tail conjugation
- ↑ Boucher Y, Doolittle WF “The role of lateral gene transfer in the evolution of isoprenoid biosynthesis pathways” Mol Microbiol 37(4):703-716, 2000
- ↑ McGarvey DJ, Croteau R “Terpenoid metabolism” Plant Cell 7:1015-1026, 1995
- ↑ Lichtenthaler HK, Schwender J, Disch A, Rohmer M. “Biosynthesis of isoprenoids in higher plant chloroplasts proceeds via a mevalonate-independent pathway” FEBS Lett 400(3):271-4, 1997
- ↑ Turner G, Gershenzon J, Nielson EE, Froehlich JE, Croteau R “Limonene synthase, the enzyme responsible for monoterpene biosynthesis in peppermint, is localized to leucoplasts of oil gland secretory cells” Plant Physiol 120(3):879-886, 1999
This category currently contains no pages or media.