Category:TP
m (→Overview) |
m (→History) |
||
| Line 18: | Line 18: | ||
===History=== | ===History=== | ||
{{Twocolumn| | {{Twocolumn| | ||
| − | The German chemist Otto Wallach proposed the construction of terpenes from isoprene units known as the ''isoprene rule'' (1887), and received [http://nobelprize.org/nobel_prizes/chemistry/laureates/1910/wallach-lecture.html the Nobel Prize in Chemistry in 1910]. | + | * The German chemist Otto Wallach proposed the construction of terpenes from isoprene units known as the ''isoprene rule'' (1887), and received [http://nobelprize.org/nobel_prizes/chemistry/laureates/1910/wallach-lecture.html the Nobel Prize in Chemistry in 1910]. |
| − | In 1930s Leopold Ruzicka rationalized the reaction mechanisms of isoprene units and proposed the ''biogenetic isoprene rule'', in which all compounds derived from ''active'' isoprene units should be included as terpenes. This active isoprene is now known as isopentenyl di- (or pyro-) phosphate (IPP). He received [http://nobelprize.org/nobel_prizes/chemistry/laureates/1939/ruzicka-lecture.html the Nobel Prize in Chemistry in 1939]. | + | * In 1930s Leopold Ruzicka rationalized the reaction mechanisms of isoprene units and proposed the ''biogenetic isoprene rule'', in which all compounds derived from ''active'' isoprene units should be included as terpenes. This active isoprene is now known as isopentenyl di- (or pyro-) phosphate (IPP). He received [http://nobelprize.org/nobel_prizes/chemistry/laureates/1939/ruzicka-lecture.html the Nobel Prize in Chemistry in 1939].<ref>[http://www.springerlink.com/content/nrmg1jpq1454663p/ Ruzicka, L "The isoprene rule and the biogenesis of terpenic compounds" ''Cell. Mol. Life Sci.'' 9(10): 357-367, 1953]</ref> |
| + | * In 1999, Rohmer et al. elucidated the non-mevalonate pathway or MEP pathway. | ||
| | | | ||
| − | ドイツの化学者オットー・ヴァラッハは、テルペンがイソプレン単位から構成されること(イソプレン則)を示し、[http://nobelprize.org/nobel_prizes/chemistry/laureates/1910/wallach-lecture.html 1910年ノーベル化学賞] | + | * ドイツの化学者オットー・ヴァラッハは、テルペンがイソプレン単位から構成されること(イソプレン則)を示し、[http://nobelprize.org/nobel_prizes/chemistry/laureates/1910/wallach-lecture.html 1910年ノーベル化学賞]を受賞しました。 |
| − | + | * 1930年代にスイスのレオポルド・ルジツカがイソプレン単位の反応機構を整理し、反応性の高いイソプレンの生体内反応による産物を全てテルペンとするイソプレン生合成則を示しました。 | |
| − | 反応性の高いイソプレンとは、イソペンテニル二リン酸 (isopentenyl pyrophosphate: IPP) | + | 反応性の高いイソプレンとは、イソペンテニル二リン酸 (isopentenyl pyrophosphate: IPP) であることが現在わかっています。 |
| − | ルジツカはこの業績により[http://nobelprize.org/nobel_prizes/chemistry/laureates/1939/ruzicka-lecture.html 1937年ノーベル化学賞] | + | ルジツカはこの業績により[http://nobelprize.org/nobel_prizes/chemistry/laureates/1939/ruzicka-lecture.html 1937年ノーベル化学賞]を受賞しました。 |
}} | }} | ||
Revision as of 14:51, 31 July 2010
Contents |
Terpenes
Overview
The word 'terpene' originates from turpentine, the distillation of resin from pine trees (Pinaceae). Chemically speaking, terpenes or terpenoids refer to all natural compounds build up from isoprene (C5, 2-methyl-1,3-butadiene) units.
For this reason, terpenes are also called isoprenoids.
Well known terpenes include myrrh from balm trees, fragrance or essential oils of herbs and roses. Biologically, terpenes function as volatile expellant of herbivores and attractant of pollinators, or less volatile bitter antifeedants. Many hormones, pheromones and signal compounds are also derived from terpenes.
History
- The German chemist Otto Wallach proposed the construction of terpenes from isoprene units known as the isoprene rule (1887), and received the Nobel Prize in Chemistry in 1910.
- In 1930s Leopold Ruzicka rationalized the reaction mechanisms of isoprene units and proposed the biogenetic isoprene rule, in which all compounds derived from active isoprene units should be included as terpenes. This active isoprene is now known as isopentenyl di- (or pyro-) phosphate (IPP). He received the Nobel Prize in Chemistry in 1939.[1]
- In 1999, Rohmer et al. elucidated the non-mevalonate pathway or MEP pathway.
- ↑ Ruzicka, L "The isoprene rule and the biogenesis of terpenic compounds" Cell. Mol. Life Sci. 9(10): 357-367, 1953
Classification
Depending on the number of isoprene units used, terpenoids are classified as follows.
- hemi-terpenes (C5)
- mono- (C10)
- sesqui- (C15)
- di- (C20)
- sester- (C25)
- tri- (C30)
- tetra- (C40)
- poly- (C5)n (n > 8)
Biosynthesis
The common precursor of terpenes is isopentenyl diphosphate (IPP) and its isomer, dimethylallyl diphosphate (DMAPP). Both are synthesized through the mevalonate pathway (MVA pathway).
| IPP | DMAPP | |
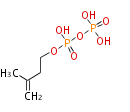
|
IPP isomerase EC5.3.3.2 |
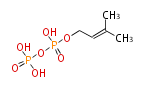
|
Mevalonate Pathway
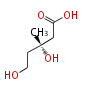
In the MVA pathway, 3 molecules of acetyl CoA are first condensed to produce 3-hydroxy-3-methyl-glutaryl CoA (HMG-CoA), which is then reduced using 2 molecules of NADPH to form 3(R)-mevalonic acid (MVA). The reduction by HMG-CoA reductase is considered the rate-limiting step of IPP biosynthesis (and therefore cholesterol biosynthesis), and the well-known drugs "statin" prescribed for hypercholesterolemia are HMG-CoA reductase inhibitors[1]. Mevalonic acid is then twice phosphorylated by 2 molecules of ATP to form IPP.
| HMG-CoA | (3R)-MVA | IPP | ||
|
HMG-CoA reductase EC1.1.1.34 |
|
kinase & decarboxylase EC2.7.4.2 & EC4.1.1.33 |

|
The plant HMG-CoA reductases are also rate-limiting and are located in the ER membrane. Upon wounding or pathogen infection they are activated to prouduce terpenes.
- ↑ Endo A “The discovery and development of HMG-CoA reductase inhibitors” J Lipid Res 33 (11): 1569–1582, 1992
Methylerythritol-phosphate Pathway
In plants, MVA is synthesized through the methylerythritol phosphate (MEP) pathway.
This pathway is also referred to as deoxyxylulose phosphate (DXP) or MVA-independent pathway, but the name "MEP" is preferred to specify the isoprenoid biosynthesis, because DXP is also known as a precursor of pyridoxal, the major form of Vitamin B6 in animal tissues (Vitamin B6 includes pyridoxal, pyridoxamine, and pyridoxine and their phosphate forms) [1].
Acetyl moiety from pyruvic acid is transferred onto D-glyceraldehyde 3-phosphate to form 1-deoxy-D-xylulose 5-phosphate. Then it undergoes a reverse aldol rearrangement in an enzyme-bound manner to form a branched chain compound, 2-C-methyl-D-erythritol 4-phosphate (MEP). MEP is attached by cytidine triphosphate (CTP) and its 2-hydroxyl position is phosphorylated, followed by a cyclization into 2-C-methyl-D-erythritol-2,4-cyclophosphate. IPP and DMAPP are produced from the cyclic anhydride in still unidentified enzymatic steps.
- ↑ Tambasco-Studart M, Titiz O, Raschle T, Forster G, Amrhein N, Fitzpatrick TB “Vitamin B6 biosynthesis in higher plants” Proc Natl Acad Sci USA 102(38):13687-13692, 2005
Terpene biosynthesis
Animals and fungi lack the MEP pathway and exclusively utilize the MVA pathway in cytosol [1]. Plants use both pathways but selectively: IPP is turned into farnesyl diphosphate (C15) in the cytosol and the cytosol/endoplasmic reticulum boundary, and the mevalonate pathway is largely responsible for sesquiterpenoids (C15), steroids (C30), and triterpenoids (C30) [2]. On the other hands, enzymes related to the MEP pathway exist in plastids, and monoterpenoids (C10), diterpenoids (C20) and carotenes (C40) are synthesized mainly there [3][4].
... reactions in plastids
... reactions in the cytosol & ER
- (h-t) ... head-to-tail conjugation
- (t-t) ... tail-to-tail conjugation
- ↑ Boucher Y, Doolittle WF “The role of lateral gene transfer in the evolution of isoprenoid biosynthesis pathways” Mol Microbiol 37(4):703-716, 2000
- ↑ McGarvey DJ, Croteau R “Terpenoid metabolism” Plant Cell 7:1015-1026, 1995
- ↑ Lichtenthaler HK, Schwender J, Disch A, Rohmer M. “Biosynthesis of isoprenoids in higher plant chloroplasts proceeds via a mevalonate-independent pathway” FEBS Lett 400(3):271-4, 1997
- ↑ Turner G, Gershenzon J, Nielson EE, Froehlich JE, Croteau R “Limonene synthase, the enzyme responsible for monoterpene biosynthesis in peppermint, is localized to leucoplasts of oil gland secretory cells” Plant Physiol 120(3):879-886, 1999
This category currently contains no pages or media.