Category:TP
m (→Biosynthesis) |
|||
| Line 1: | Line 1: | ||
| − | = | + | ==Biosynthesis== |
| − | + | ||
{{Twocolumn| | {{Twocolumn| | ||
| − | The | + | The common precursor of terpenes is [[BMFYB4PHr002|isopentenyl diphosphate (IPP)]] and its isomer, [[BMFYB4PHr003|dimethylallyl diphosphate (DMAPP)]]. Both are synthesized through the mevalonate pathway (MVA pathway). |
| − | + | ||
| − | + | ||
| − | + | ||
| | | | ||
| − | + | テルペンに共通する前駆体はイソペンテニル二リン酸 (IPP) とその異性体ジメチルアリル二リン酸 (DMAPP) である。両者はメバロン酸経路で合成される。 | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
}} | }} | ||
| − | === | + | <center> |
| + | {| style="text-align:center" | ||
| + | | [[BMFYB4PHr002|IPP]] || || [[BMFYB4PHr003|DMAPP]] | ||
| + | |- | ||
| + | | [[Image:BMFYB4PHr002.png]] | ||
| + | | IPP isomerase<br/><math>\longleftrightarrow</math><br/>[[Enzyme:5.3.3.2|EC5.3.3.2]] | ||
| + | | [[Image:BMFYB4PHr003.png]] | ||
| + | |} | ||
| + | </center> | ||
| + | |||
| + | ===Mevalonate Pathway=== | ||
{{Twocolumn| | {{Twocolumn| | ||
| − | + | In the MVA pathway, 3 molecules of acetyl CoA are first condensed to produce 3-hydroxy-3-methyl-glutaryl CoA (HMG-CoA), which is then reduced using 2 molecules of NADPH to form 3(''R'')-mevalonic acid (MVA). | |
| − | + | The reduction by HMG-CoA reductase is considered the rate-limiting step of IPP biosynthesis (and therefore cholesterol biosynthesis), and the well-known drugs "statin" prescribed for hypercholesterolemia are HMG-CoA reductase inhibitors<ref>Endo A “The discovery and development of HMG-CoA reductase inhibitors” J Lipid Res 33 (11): 1569–1582, 1992</ref>. | |
| + | Mevalonic acid is then twice phosphorylated by 2 molecules of ATP to form IPP. | ||
| | | | ||
| − | + | メバロン酸経路では3分子のアセチルCoAが重合してヒドロキシメチルグルタリルCoA (HMG-CoA) を作り、それが2分子のNADPHにより還元されてメバロン酸 (MVA) となる。このHMG-CoA 還元酵素による反応はIPP合成 (すなわちコレステロール合成) の律速段階とされ、高コレステロール血症の治療薬スタチンは、このHMG-CoA還元酵素阻害剤である。 | |
| − | + | メバロン酸は2分子のATPを用いて2度リン酸化され、IPPとなる。 | |
| − | + | ||
| − | + | ||
}} | }} | ||
| + | |||
| + | <center> | ||
| + | {| style="text-align:center" | ||
| + | | [[BMFYB5CAa008|HMG-CoA]] || || [[BMFYB5CAr004|(3''R'')-MVA]] || || IPP | ||
| + | |- | ||
| + | | [[Image:BMFYB5CAa008.png]] | ||
| + | | HMG-CoA reductase<br/><math>\longrightarrow</math><br/>[[Enzyme:1.1.1.34|EC1.1.1.34]] | ||
| + | | [[Image:BMFYB5CAr004.png]] | ||
| + | | kinase & decarboxylase<br/><math>\rightarrow \rightarrow</math><br/>[[Enzyme:2.7.4.2| EC2.7.4.2]] & [[Enzyme:4.1.1.33|EC4.1.1.33]] | ||
| + | | [[Image:BMFYB4PHr002.png]] | ||
| + | |} | ||
| + | </center> | ||
<references/> | <references/> | ||
| − | === | + | ===Methylerythritol-phosphate Pathway=== |
{{Twocolumn| | {{Twocolumn| | ||
| − | + | In plants, MVA is synthesized through the methylerythritol phosphate (MEP) pathway. | |
| − | + | This pathway is also referred to as deoxyxylulose phosphate (DXP) or MVA-independent pathway, but the name "MEP" is preferred to specify the isoprenoid biosynthesis, because DXP is also known as a precursor of pyridoxal, the major form of Vitamin B6 in animal tissues (Vitamin B6 includes pyridoxal, pyridoxamine, and pyridoxine and their phosphate forms) <ref>Tambasco-Studart M, Titiz O, Raschle T, Forster G, Amrhein N, Fitzpatrick TB “Vitamin B6 biosynthesis in higher plants” Proc Natl Acad Sci USA 102(38):13687-13692, 2005</ref>. | |
| − | + | <br/> | |
| − | + | Acetyl moiety from pyruvic acid is transferred onto D-glyceraldehyde 3-phosphate to form 1-deoxy-D-xylulose 5-phosphate. Then it undergoes a reverse aldol rearrangement in an enzyme-bound manner to form a branched chain compound, 2-C-methyl-D-erythritol 4-phosphate (MEP). MEP is attached by cytidine triphosphate (CTP) and its 2-hydroxyl position is phosphorylated, followed by a cyclization into 2-C-methyl-D-erythritol-2,4-cyclophosphate. IPP and DMAPP are produced from the cyclic anhydride in still unidentified enzymatic steps. | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| | | | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
}} | }} | ||
| − | == | + | <references/> |
| + | ===Terpene biosynthesis=== | ||
{{Twocolumn| | {{Twocolumn| | ||
| − | + | Animals and fungi lack the MEP pathway and exclusively utilize the MVA pathway in cytosol <ref>Boucher Y, Doolittle WF “The role of lateral gene transfer in the evolution of isoprenoid biosynthesis pathways” Mol Microbiol 37(4):703-716, 2000</ref>. Plants use both pathways but selectively: IPP is turned into farnesyl diphosphate (C15) in the cytosol and the cytosol/endoplasmic reticulum boundary, and the mevalonate pathway is largely responsible for sesquiterpenoids (C15), steroids (C30), and triterpenoids (C30) <ref>McGarvey DJ, Croteau R “Terpenoid metabolism” Plant Cell 7:1015-1026, 1995</ref>. On the other hands, enzymes related to the MEP pathway exist in plastids, and monoterpenoids (C10), diterpenoids (C20) and carotenes (C40) are synthesized mainly there <ref>Lichtenthaler HK, Schwender J, Disch A, Rohmer M. “Biosynthesis of isoprenoids in higher plant chloroplasts proceeds via a mevalonate-independent pathway” FEBS Lett 400(3):271-4, 1997</ref><ref>Turner G, Gershenzon J, Nielson EE, Froehlich JE, Croteau R “Limonene synthase, the enzyme responsible for monoterpene biosynthesis in peppermint, is localized to leucoplasts of oil gland secretory cells” Plant Physiol 120(3):879-886, 1999</ref>. | |
| | | | ||
| − | + | ||
}} | }} | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* (h-t) ... head-to-tail conjugation | * (h-t) ... head-to-tail conjugation | ||
| Line 119: | Line 106: | ||
|} | |} | ||
| − | + | <references/> | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
Revision as of 22:31, 22 July 2010
Contents |
Biosynthesis
The common precursor of terpenes is isopentenyl diphosphate (IPP) and its isomer, dimethylallyl diphosphate (DMAPP). Both are synthesized through the mevalonate pathway (MVA pathway).
| IPP | DMAPP | |
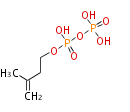
|
IPP isomerase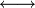 EC5.3.3.2 |
|
Mevalonate Pathway
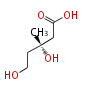
In the MVA pathway, 3 molecules of acetyl CoA are first condensed to produce 3-hydroxy-3-methyl-glutaryl CoA (HMG-CoA), which is then reduced using 2 molecules of NADPH to form 3(R)-mevalonic acid (MVA). The reduction by HMG-CoA reductase is considered the rate-limiting step of IPP biosynthesis (and therefore cholesterol biosynthesis), and the well-known drugs "statin" prescribed for hypercholesterolemia are HMG-CoA reductase inhibitors[1]. Mevalonic acid is then twice phosphorylated by 2 molecules of ATP to form IPP.
| HMG-CoA | (3R)-MVA | IPP | ||
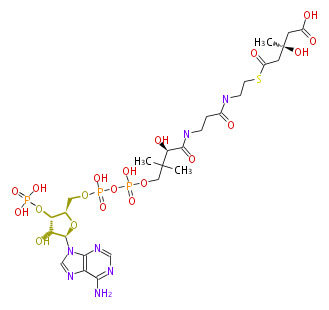
|
HMG-CoA reductase EC1.1.1.34 |
|
kinase & decarboxylase EC2.7.4.2 & EC4.1.1.33 |

|
- ↑ Endo A “The discovery and development of HMG-CoA reductase inhibitors” J Lipid Res 33 (11): 1569–1582, 1992
Methylerythritol-phosphate Pathway
In plants, MVA is synthesized through the methylerythritol phosphate (MEP) pathway.
This pathway is also referred to as deoxyxylulose phosphate (DXP) or MVA-independent pathway, but the name "MEP" is preferred to specify the isoprenoid biosynthesis, because DXP is also known as a precursor of pyridoxal, the major form of Vitamin B6 in animal tissues (Vitamin B6 includes pyridoxal, pyridoxamine, and pyridoxine and their phosphate forms) [1].
Acetyl moiety from pyruvic acid is transferred onto D-glyceraldehyde 3-phosphate to form 1-deoxy-D-xylulose 5-phosphate. Then it undergoes a reverse aldol rearrangement in an enzyme-bound manner to form a branched chain compound, 2-C-methyl-D-erythritol 4-phosphate (MEP). MEP is attached by cytidine triphosphate (CTP) and its 2-hydroxyl position is phosphorylated, followed by a cyclization into 2-C-methyl-D-erythritol-2,4-cyclophosphate. IPP and DMAPP are produced from the cyclic anhydride in still unidentified enzymatic steps.
- ↑ Tambasco-Studart M, Titiz O, Raschle T, Forster G, Amrhein N, Fitzpatrick TB “Vitamin B6 biosynthesis in higher plants” Proc Natl Acad Sci USA 102(38):13687-13692, 2005
Terpene biosynthesis
Animals and fungi lack the MEP pathway and exclusively utilize the MVA pathway in cytosol [1]. Plants use both pathways but selectively: IPP is turned into farnesyl diphosphate (C15) in the cytosol and the cytosol/endoplasmic reticulum boundary, and the mevalonate pathway is largely responsible for sesquiterpenoids (C15), steroids (C30), and triterpenoids (C30) [2]. On the other hands, enzymes related to the MEP pathway exist in plastids, and monoterpenoids (C10), diterpenoids (C20) and carotenes (C40) are synthesized mainly there [3][4].
- (h-t) ... head-to-tail conjugation
- (t-t) ... tail-to-tail conjugation
- ↑ Boucher Y, Doolittle WF “The role of lateral gene transfer in the evolution of isoprenoid biosynthesis pathways” Mol Microbiol 37(4):703-716, 2000
- ↑ McGarvey DJ, Croteau R “Terpenoid metabolism” Plant Cell 7:1015-1026, 1995
- ↑ Lichtenthaler HK, Schwender J, Disch A, Rohmer M. “Biosynthesis of isoprenoids in higher plant chloroplasts proceeds via a mevalonate-independent pathway” FEBS Lett 400(3):271-4, 1997
- ↑ Turner G, Gershenzon J, Nielson EE, Froehlich JE, Croteau R “Limonene synthase, the enzyme responsible for monoterpene biosynthesis in peppermint, is localized to leucoplasts of oil gland secretory cells” Plant Physiol 120(3):879-886, 1999
This category currently contains no pages or media.