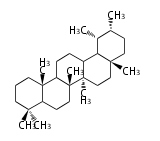
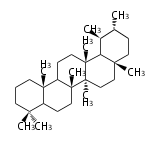
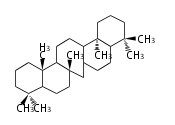
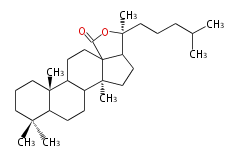Category:TP3
(→Design of Tri-terpene ID numbers ID番号の設計) |
|||
| Line 1: | Line 1: | ||
==Triterpene (C30) Classes== | ==Triterpene (C30) Classes== | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
==Ring configuration== | ==Ring configuration== | ||
| Line 54: | Line 46: | ||
|} | |} | ||
</center> | </center> | ||
| + | |||
| + | |||
| + | ==Biosynthesis== | ||
| + | {{Twocolumn| | ||
| + | The starting point is 2,3-oxidosqualene, which is formed by joining two FPPs tail-to-tail. | ||
| + | This molecule undergoes cyclization with Wagner-Meerwein shift to form taxa-dependent precursors. | ||
| + | ; animals, fungi, and yeast | ||
| + | : 2,3-oxidosqualene → lanosterol | ||
| + | ; plants (including algae) | ||
| + | : 2,3-oxidosqualene → cycloartenol, dammarane | ||
| + | ; bacteria | ||
| + | : 2,3-oxidosqualene → hopene | ||
| + | | | ||
| + | 始まりとなる物質は2,3-オキシドスクアレンで、二分子のファルネシル二リン酸が末尾どうしで結合して作られます。 | ||
| + | これが環化して骨格転位反応 (Wagner-Meerwein shift) を起こし、種ごとに異なる前駆体を形成します。 | ||
| + | ; 動物、カビ、酵母 | ||
| + | : 2,3-オキシドスクアレン → ラノステロール | ||
| + | ; 植物 (および藻類) | ||
| + | : 2,3-オキシドスクアレン → シクロアルテノール、ダンマラン | ||
| + | ; バクテリア | ||
| + | : 2,3-オキシドスクアレン → ホペン | ||
| + | }} | ||
| + | |||
| + | <ref>Ohyama K, Suzuki M, Kikuchi J, Saito K, Muranaka T “Dual biosynthetic pathways to phytosterol via cycloartenol and lanosterol in Arabidopsis” Proc Natl Acad Sci USA 106(3):725-730, 2009</ref> | ||
==Design of Tri-terpene ID numbers ID番号の設計== | ==Design of Tri-terpene ID numbers ID番号の設計== | ||
Revision as of 14:01, 4 August 2010
Contents |
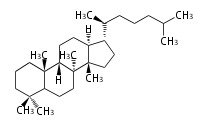
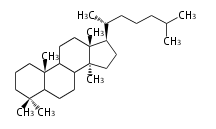
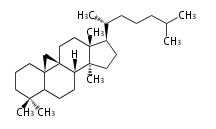
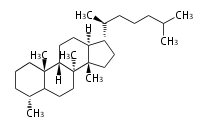
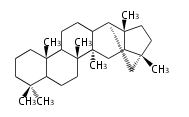
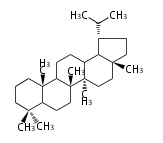
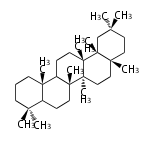
Triterpene (C30) Classes
Ring configuration
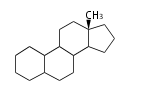
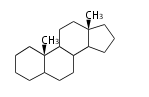
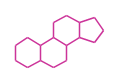
The basic structure is 4 carbon rings, cyclopenta[a]phenanthrene, gonane, or sterane. The rings B/C are always trans in all natural steroids. If the rings C/D are trans, it is called gonane. If its stereochemistry is unspecified, it is called sterane. Most steroids take gonane form, but in cardenolides and bufanolides, the rings C/D are cis.
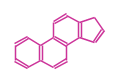 |
|
| Cyclopenta[a]phenanthrene | Gonane |
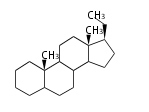
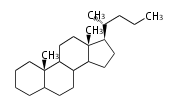
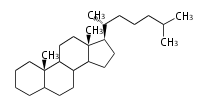
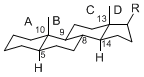
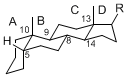
The majority of steroids have methyl groups sticking out from the bridgehead positions C-10 and C-13. When these methyl groups (or hydrogens) stand above the plane, they are called β-configuration. Those below the plane are called α-configuration. If the configuration at any site is unknown, it is indicated as ξ (Greek Xi). By default, hydrogen atoms or substituents at the positions C-8, 9, 10, 13, and 14 are assumed to be 8β, 9α, 10β, 13β, and 14α configurations. C-5 is a special position, because there are as many 5α steroids as 5β are.
 |
 |
|
| cholestane backbone | 5α-configuration | 5β-configuration |
Biosynthesis
The starting point is 2,3-oxidosqualene, which is formed by joining two FPPs tail-to-tail. This molecule undergoes cyclization with Wagner-Meerwein shift to form taxa-dependent precursors.
- animals, fungi, and yeast
- 2,3-oxidosqualene → lanosterol
- plants (including algae)
- 2,3-oxidosqualene → cycloartenol, dammarane
- bacteria
- 2,3-oxidosqualene → hopene
Design of Tri-terpene ID numbers ID番号の設計
12-DIGIT
| T | P | 3 | x | y | y | r | h | g | n | c | c |
- x ... species information
| Symbol at x | Kingdom | Phyla | Examples |
|---|---|---|---|
| I | Animalia | Arthropoda (Insects, crabs) | ecdysteroids |
| V | Chordate (Vertebrates) | sex steroids, corticosteroids, anabolic steroids | |
| O | Others | marine steroids | |
| P | Plantae | Phytosterols | lanosterols, cholesterols, brassinolides |
| S | Saponins | saponins | |
| F | Fungi | ergosterols | ergosterols |
| B | Bacteria | bacterial sterols | hopanoids |
- y ... backbone structure (母核構造)
- r ... number of major rings (環構造数)
Click above categories to see details.
- h ... hydroxylation pattern (水酸基数)
Click above categories to see details.
- g ... glycosylation pattern(糖修飾パターン)
Click above categories to see details.
- n ... number of sugars (修飾糖数)
Click above categories to see details.
- c ... serial number (通し番号)
Cite error:
<ref> tags exist, but no <references/> tag was found
This category currently contains no pages or media.