Category:FL
| Line 1: | Line 1: | ||
| − | =Flavonoid= | + | ==FL: Flavonoid== |
| − | Flavonoid is a class of plant secondary metabolites that have two benzene rings (each called A-ring and B-ring) connected by a chain of three carbons (Figure 1). [[Image:FL.gif|thumb|150px|right| | + | ===Structural Characteristics=== |
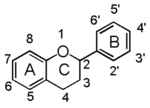
| + | Flavonoid is a class of plant secondary metabolites that have two benzene rings (each called A-ring and B-ring) connected by a chain of three carbons (Figure 1). [[Image:FL.gif|thumb|150px|right|Figure 1: The Backbone of Flavonoid Structure]] | ||
| + | The carbon chain, corresponding to the numbers 2,3,4 in Figure 1, is linked to a hydroxyl group in the A-ring to form the C-ring. The class of flavonoids are usually determined by the modification pattern of the C-ring (Table 1). | ||
| − | <table> | + | <table border=1> |
<tr> | <tr> | ||
| − | <td>[[Image:Fl1c.gif| | + | <th>names</th> |
| − | <td>[[Image:Fl2.gif| | + | <th>structure</th> |
| − | <td>[[Image:Fl3.gif| | + | <th>class</th> |
| − | </tr> | + | <th>description</th> |
| − | <tr> | + | </tr> |
| − | <td>[[Image:Fl4.gif| | + | <tr> |
| − | <td>[[Image:Fl5.gif| | + | <td align=center>Aurones<br>and<br>Chalcones</td> |
| − | <td>[[Image:Fl6.gif| | + | <td>[[Image:Fl1c.gif|100px]]</td> |
| − | </tr> | + | <td align=center>[[:Category:FL1|FL1]]</td> |
| − | <tr> | + | <td>The C-ring is absent (chalcones) or a 5-member ring (aurones). |
| − | <td>[[Image:Fl7.gif| | + | </td> |
| − | <td>[[Image:Fli.gif| | + | </tr> |
| − | <td>[[Image:Fln.gif| | + | <tr> |
| − | </tr></table> | + | <td align=center>Flavanone</td> |
| + | <td>[[Image:Fl2.gif|100px]]</td> | ||
| + | <td align=center>[[:Category:FL2|FL2]]</td> | ||
| + | <td> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td align=center>Flavone</td> | ||
| + | <td>[[Image:Fl3.gif|100px]]</td> | ||
| + | <td align=center>[[:Category:FL3|FL3]]</td> | ||
| + | <td> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td align=center>Dihydroflavonol</td> | ||
| + | <td>[[Image:Fl4.gif|100px]]</td> | ||
| + | <td align=center>[[:Category:FL4|FL4]]</td> | ||
| + | <td>Because many flavonols (FL5) are hydroxylated at their position 3, they form a different category in our classification.</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td align=center>Flavonol</td> | ||
| + | <td>[[Image:Fl5.gif|100px]]</td> | ||
| + | <td align=center>[[:Category:FL5|FL5]]</td> | ||
| + | <td> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td align=center>Flavan</td> | ||
| + | <td>[[Image:Fl6.gif|100px]]</td> | ||
| + | <td align=center>[[:Category:FL6|FL6]]</td> | ||
| + | <td> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td align=center>Anthocyanin</td> | ||
| + | <td>[[Image:Fl7.gif|100px]]</td> | ||
| + | <td align=center>[[:Category:FL7|FL7]]</td> | ||
| + | <td> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td align=center>Isoflavonoid</td> | ||
| + | <td>[[Image:Fli.gif|100px]]</td> | ||
| + | <td align=center>[[:Category:FLI|FLI]]</td> | ||
| + | <td> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td align=center>Neoflavonoid</td> | ||
| + | <td>[[Image:Fln.gif|70px]]</td> | ||
| + | <td align=center>[[:Category:FLN|FLN]]</td> | ||
| + | <td> | ||
| + | </td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | ===Biosynthesis=== | ||
Flavonoid is synthesized through the phenylpropanoid-acetate pathway in all higher plants. It is responsible for many biological activities including pigments, anti-oxidative or anti-allergic agents, and signaling elements in nodule formation. Some of them are quite familiar in our daily life. | Flavonoid is synthesized through the phenylpropanoid-acetate pathway in all higher plants. It is responsible for many biological activities including pigments, anti-oxidative or anti-allergic agents, and signaling elements in nodule formation. Some of them are quite familiar in our daily life. | ||
Revision as of 16:47, 2 March 2008
FL: Flavonoid
Structural Characteristics
Flavonoid is a class of plant secondary metabolites that have two benzene rings (each called A-ring and B-ring) connected by a chain of three carbons (Figure 1).The carbon chain, corresponding to the numbers 2,3,4 in Figure 1, is linked to a hydroxyl group in the A-ring to form the C-ring. The class of flavonoids are usually determined by the modification pattern of the C-ring (Table 1).
| names | structure | class | description |
|---|---|---|---|
| Aurones and Chalcones |
100px | FL1 | The C-ring is absent (chalcones) or a 5-member ring (aurones). |
| Flavanone | 100px | FL2 | |
| Flavone | 100px | FL3 | |
| Dihydroflavonol | 100px | FL4 | Because many flavonols (FL5) are hydroxylated at their position 3, they form a different category in our classification. |
| Flavonol | 100px | FL5 | |
| Flavan | 100px | FL6 | |
| Anthocyanin | 100px | FL7 | |
| Isoflavonoid | 100px | FLI | |
| Neoflavonoid | 70px | FLN |
Biosynthesis
Flavonoid is synthesized through the phenylpropanoid-acetate pathway in all higher plants. It is responsible for many biological activities including pigments, anti-oxidative or anti-allergic agents, and signaling elements in nodule formation. Some of them are quite familiar in our daily life.
Familiar examples.
anthocyanin (blueberry), isoflavone (soybean), rutin (soba noodle), catechin (tea), flavan-diol (tea), naringeninchalcone (tomato), polyphenol (wine, cacao)
Subcategories
This category has the following 10 subcategories, out of 10 total.