FL7AAIGL0025
From Metabolomics.JP
(Difference between revisions)
| (3 intermediate revisions by one user not shown) | |||
| Line 1: | Line 1: | ||
| + | {{Hierarchy|{{PAGENAME}}}} | ||
| + | |||
{{Metabolite | {{Metabolite | ||
| − | |SysName=Malvidin 3-O-[6-O-[4-O-[4-O-(6-O-caffeoyl-beta-D-glucopyranosyl)-p-coumaroyl]-alpha-L-rhamnosyl]-beta-D-glucopyranoside]-5-O-beta-D-glucopyranoside | + | |SysName=Malvidin 3-O- [ 6-O- [ 4-O- [ 4-O- (6-O-caffeoyl-beta-D-glucopyranosyl) -p-coumaroyl ] -alpha-L-rhamnosyl ] -beta-D-glucopyranoside ] -5-O-beta-D-glucopyranoside |
| − | |Common Name=&&Malvidin 3-O-[6-O-[4-O-[4-O-(6-O-caffeoyl-beta-D-glucopyranosyl)-p-coumaroyl]-alpha-L-rhamnosyl]-beta-D-glucopyranoside]-5-O-beta-D-glucopyranoside&& | + | |Common Name=&&Malvidin 3-O- [ 6-O- [ 4-O- [ 4-O- (6-O-caffeoyl-beta-D-glucopyranosyl) -p-coumaroyl ] -alpha-L-rhamnosyl ] -beta-D-glucopyranoside ] -5-O-beta-D-glucopyranoside&& |
|CAS=210050-04-7 | |CAS=210050-04-7 | ||
|KNApSAcK=C00011074 | |KNApSAcK=C00011074 | ||
}} | }} | ||
Latest revision as of 09:00, 22 September 2008
| Flavonoid Top | Molecule Index | Author Index | Journals | Structure Search | Food | New Input |
Upper classes : FL Flavonoid : FL7 Anthocyani(di)n : FL7A Anthocyani(di)n : FL7AAI Malvidin (52 pages) : FL7AAIGL Anthocyanin (3-Glucoside related) (40 pages) : FL7AAIGL0 Normal (38 pages)
| IDs and Links | |
|---|---|
| LipidBank | [1] |
| LipidMaps | [2] |
| CAS | 210050-04-7 |
| KEGG | {{{KEGG}}} |
| KNApSAcK | |
| CDX file | |
| MOL file | FL7AAIGL0025.mol |
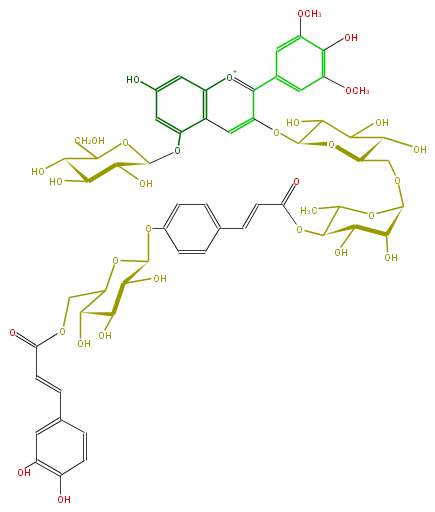
| Malvidin 3-O- [ 6-O- [ 4-O- [ 4-O- (6-O-caffeoyl-beta-D-glucopyranosyl) -p-coumaroyl ] -alpha-L-rhamnosyl ] -beta-D-glucopyranoside ] -5-O-beta-D-glucopyranoside | |
|---|---|
| |
| Structural Information | |
| Systematic Name | Malvidin 3-O- [ 6-O- [ 4-O- [ 4-O- (6-O-caffeoyl-beta-D-glucopyranosyl) -p-coumaroyl ] -alpha-L-rhamnosyl ] -beta-D-glucopyranoside ] -5-O-beta-D-glucopyranoside |
| Common Name |
|
| Symbol | |
| Formula | C59H67O31 |
| Exact Mass | 1271.366630426 |
| Average Mass | 1272.1446799999999 |
| SMILES | c(c(O)1)(ccc(C=CC(OCC(O2)C(O)C(O)C(C2Oc(c9)ccc(c9) |
| Physicochemical Information | |
| Melting Point | |
| Boiling Point | |
| Density | |
| Optical Rotation | |
| Reflactive Index | |
| Solubility | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | |
| Chromatograms | |
Species Information
| Species-Flavonoid Relationship Reported | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|