Category:TP3P
(→Phytosterols) |
|||
| Line 14: | Line 14: | ||
<center> | <center> | ||
{| | {| | ||
| + | ! style="background-color:#fdd"| animal sterols | ||
| + | ! colspan="2" style="background-color:#dfd"| plant sterols | ||
|- | |- | ||
| [[Image:cholestane.png]] || [[Image:campestane.png]] || [[Image:stigmastane.png]] | | [[Image:cholestane.png]] || [[Image:campestane.png]] || [[Image:stigmastane.png]] | ||
| Line 27: | Line 29: | ||
Soybean ([[Species:Glycine|''Glycine max'']], [[:Category:Fabaceae|Fabaceae]]) is a rich source of phytosterols (about 0.1% of its weight), and is used for semi-synthesis of medicinal steroids <ref>Yamaya A, Endo Y, Fujimoto K, Kitamura K. (2006) “Effects of genetic variability and planting location on the phytosterol content and composition in soybean seeds” Food Chem 102(4): 1071-1075</ref>. Since dietary phytosterols reduce cholesterol levels, they are used as food additives such as for margarine <ref>Schiepers OJ, de Groot RH, van Boxtel MP, Jolles J, de Jong A, Lütjohann D, Plat J, Mensink RP (2009) “Consuming functional foods enriched with plant sterol or stanol esters for 85 weeks does not affect neurocognitive functioning or mood in statin-treated hypercholesterolemic individuals” J Nutr 139(7):1368-1373</ref>. Vitamin D is a family of sterol metabolites generated photochemically in our skin by UV irradiation. | Soybean ([[Species:Glycine|''Glycine max'']], [[:Category:Fabaceae|Fabaceae]]) is a rich source of phytosterols (about 0.1% of its weight), and is used for semi-synthesis of medicinal steroids <ref>Yamaya A, Endo Y, Fujimoto K, Kitamura K. (2006) “Effects of genetic variability and planting location on the phytosterol content and composition in soybean seeds” Food Chem 102(4): 1071-1075</ref>. Since dietary phytosterols reduce cholesterol levels, they are used as food additives such as for margarine <ref>Schiepers OJ, de Groot RH, van Boxtel MP, Jolles J, de Jong A, Lütjohann D, Plat J, Mensink RP (2009) “Consuming functional foods enriched with plant sterol or stanol esters for 85 weeks does not affect neurocognitive functioning or mood in statin-treated hypercholesterolemic individuals” J Nutr 139(7):1368-1373</ref>. Vitamin D is a family of sterol metabolites generated photochemically in our skin by UV irradiation. | ||
| | | | ||
| − | + | 大豆 ([[Species:Glycine|''Glycine max'']], [[:Category:Fabaceae|Fabaceae]]) は植物ステロールが豊富で (重量のおよそ0.1%)、医薬品のステロイド合成用に使われます。 | |
| + | 植物ステロールを摂取するとコレステロール値を下げる効果があるので、植物ステロールを添加したマーガリンなども市販されています。 | ||
| + | ビタミンDもステロールの一種で、これは紫外線にあたることで皮膚内で合成されます。 | ||
}} | }} | ||
Revision as of 15:38, 3 August 2010
Contents |
Phytosterols
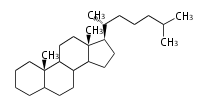
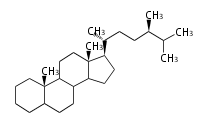
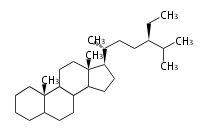
Phytosterols contain extra methyl or ethyl group in the C-17 side chain.
Most common phytosterols are campesterol, β-sitosterol, and stigmasterol. They occur both free and as 3-glucosides and all essential for membranes in higher plant. Less common are α-spinasterol (an isomer of stigmasterol) in spinach (Spinacia oleracea L., Chenopodiaceae) and alfalfa (Medicago sativa L., Fabaceae).
Some sterols are confined in lower plants. Ergosterols are found only in yeast and fungi, fucosterols are found in brown algae (Phaeophyceae) and in some higher plants, e.g. coconut (Cocos nucifera, Arecaceae.
| animal sterols | plant sterols | |
|---|---|---|
 |
 |
|
| cholestane (cholesterol backbone) |
campestane (campesterol backbone) |
stigmastane (β-sito and stigmasterol backbone) |
Phytosterols for Health
Soybean (Glycine max, Fabaceae) is a rich source of phytosterols (about 0.1% of its weight), and is used for semi-synthesis of medicinal steroids [1]. Since dietary phytosterols reduce cholesterol levels, they are used as food additives such as for margarine [2]. Vitamin D is a family of sterol metabolites generated photochemically in our skin by UV irradiation.
- ↑ Yamaya A, Endo Y, Fujimoto K, Kitamura K. (2006) “Effects of genetic variability and planting location on the phytosterol content and composition in soybean seeds” Food Chem 102(4): 1071-1075
- ↑ Schiepers OJ, de Groot RH, van Boxtel MP, Jolles J, de Jong A, Lütjohann D, Plat J, Mensink RP (2009) “Consuming functional foods enriched with plant sterol or stanol esters for 85 weeks does not affect neurocognitive functioning or mood in statin-treated hypercholesterolemic individuals” J Nutr 139(7):1368-1373
Cardenolides (C23)
The backbone of the foxglove Digitalis (Plantaginaceae) toxins with the androstane skeleton with a γ-lactone ring at C-17. Notable characters are its 14β-configuration in opposition to other steroids (the rings C/D are cis), and the 20(22)-double bond.
Since Withering's report in 1785 [1], cardenolides have been extremely valuable clinically for congestive heart failure. However, they are remarkably nontoxic to Lepidoptera, especially some species of Danaid butterfly [2]
- ↑ Tröhler U (2007) Withering's 1785 appeal for caution when reporting on a new medicine. J R Soc Med 100(3): 155–156
- ↑ Mebs D, Reuss E, Schneider M (2005) "Studies on the cardenolide sequestration in African milkweed butterflies (Danaidae)" Toxicon 45(5):581-584
Distribution
Cardenolides occur principally in the related families of Apocynaceae (including Asclepiadaceae). In the milkweed genus Asclepias, these compounds are secreted in the latex and almost every species contains these toxins. Other plant families such as Brassicaceae, Moraceae, Scrophulariaceae, and some monocotyledons (e.g. Urginea in Liliaceae) contain cardenolides.
The 5α-configuration is found in Asclepias (e.g. aspecioside), whereas 5β-configuration is common in the foxglove Digitalis purpurea, Plantaginaceae (e.g. digoxin). In some Asclepias, sugars are attached to the two hydroxyl groups at C-2 and C-3 position to form cyclic bridges (e.g. asclepin, calactin).
Withanolides (C28)
Withanolides were found in the root of Withania Somnifera, also known as Indian ginseng. Its backbone is a highly oxygenated ergostane with a γ-lactone ring linking C-22 and C-26 [1]. The configuration of C-22 is usually R.
Brassinolides (C28)
Brassinolides are plant growth-promoting hormones isolated originally from Brassica napus (rape). Its backbone is a highly oxygenated ergostane with the oxygen-expanded B-ring (ε-lactone). This lactone is not essential for plant growth activity (e.g. castasterone) but the 22R, 23R-diol are. The configurations of C-2,3 and 5 are α in brassinolides whereas they are β in ecdysteroids.
Cite error:
<ref> tags exist, but no <references/> tag was found
This category currently contains no pages or media.