Category:FL
From Metabolomics.JP
(Difference between revisions)
m (1 revision(s)) |
|||
| Line 1: | Line 1: | ||
=Flavonoid= | =Flavonoid= | ||
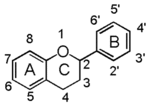
| − | Flavonoid is a class of plant secondary metabolites that have two benzene rings (each called A-ring and B-ring) connected by a chain of three carbons (Figure 1). The carbon chain, corresponding to the numbers 2,3,4 in Figure 1, can link to a hydroxyl group in the A-ring to form the C-ring. Flavonoid is structurally classified by the modification pattern of this C-ring. | + | Flavonoid is a class of plant secondary metabolites that have two benzene rings (each called A-ring and B-ring) connected by a chain of three carbons (Figure 1). [[Image:FL.gif|thumb|150px|right|Fig.1 The Backbone of Flavonoid Structure]] The carbon chain, corresponding to the numbers 2,3,4 in Figure 1, can link to a hydroxyl group in the A-ring to form the C-ring. Flavonoid is structurally classified by the modification pattern of this C-ring. |
Flavonoid is synthesized through the phenylpropanoid-acetate pathway in all higher plants. It is responsible for many biological activities including pigments, anti-oxidative or anti-allergic agents, and signaling elements in nodule formation. Some of them are quite familiar in our daily life. | Flavonoid is synthesized through the phenylpropanoid-acetate pathway in all higher plants. It is responsible for many biological activities including pigments, anti-oxidative or anti-allergic agents, and signaling elements in nodule formation. Some of them are quite familiar in our daily life. | ||
Revision as of 12:59, 2 March 2008
Flavonoid
Flavonoid is a class of plant secondary metabolites that have two benzene rings (each called A-ring and B-ring) connected by a chain of three carbons (Figure 1). The carbon chain, corresponding to the numbers 2,3,4 in Figure 1, can link to a hydroxyl group in the A-ring to form the C-ring. Flavonoid is structurally classified by the modification pattern of this C-ring.Flavonoid is synthesized through the phenylpropanoid-acetate pathway in all higher plants. It is responsible for many biological activities including pigments, anti-oxidative or anti-allergic agents, and signaling elements in nodule formation. Some of them are quite familiar in our daily life.
Familiar examples.
anthocyanin (blueberry), isoflavone (soybean), rutin (soba noodle), catechin (tea), flavan-diol (tea), naringeninchalcone (tomato), polyphenol (wine, cacao)
Subcategories
This category has the following 10 subcategories, out of 10 total.