Licoricesaponin E2
From Metabolomics.JP
(Difference between revisions)
(→Spectroscopic Data) |
|||
| Line 24: | Line 24: | ||
| '''IR''' (KBr)|| 3400-3000 (br), 2929, 1780, 1724, 1645, 1385, 1010 cm<sup>-1</sup> | | '''IR''' (KBr)|| 3400-3000 (br), 2929, 1780, 1724, 1645, 1385, 1010 cm<sup>-1</sup> | ||
|- | |- | ||
| − | | '''<sup>1</sup>H-NMR''' (C<sub>5</sub>D<sub>5</sub>N+D<sub>2</sub>O, 500 MHz)|| 0.79 (s, | + | | '''<sup>1</sup>H-NMR''' (C<sub>5</sub>D<sub>5</sub>N+D<sub>2</sub>O, 500 MHz)|| 0.79 (s, CH<sub>3</sub>), 1.06 (s, CH<sub>3</sub>), 1.21 (s, CH<sub>3</sub>), 1.22 (s, CH<sub>3</sub>), 1.34 (s, CH<sub>3</sub>), 1.40 (s, CH<sub>3</sub>), 1.42 (s, CH<sub>3</sub>), , 3.02 (brd, ''J''=''ca.'' 12.0 Hz, H-18), 3.35 (dd, ''J''=4.4. 11.2 Hz, H-3), 5.02 (d, ''J''=7.6 Hz, H-1 of GlcUA I), 5.38 (d, ''J''=7.6 Hz, H-1 of GlcUA II), 5.94 (s, H-12) |
|} | |} | ||
{| class="wikitable" style="width:80%" | {| class="wikitable" style="width:80%" | ||
Revision as of 12:14, 9 February 2010
Upper classes
| IDs and Links | |
|---|---|
| LipidBank | [1] |
| LipidMaps | [2] |
| CAS | 119417-96-8 |
| KEGG | {{{KEGG}}} |
| KNApSAcK | |
| CDX file | |
| MOL file | Licoricesaponin E2.mol |
| Licoricesaponin E2 | |
|---|---|
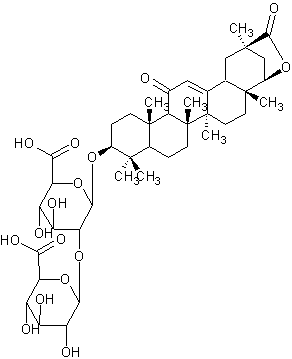
| |
| Structural Information | |
| Systematic Name | (3beta,20beta,22beta)-22,29-Epoxy-11,29-dioxoolean-12-en-3-yl 2-O-beta-D-glucopyranuronosyl-beta-D-glucopyranosiduronic acid |
| Common Name |
|
| Symbol | |
| Formula | C42H60O16 |
| Exact Mass | 820.388135872 |
| Average Mass | 820.9162 |
| SMILES | C(O1)(OC(C8(C)C)CCC(C83)(C(C7=O)C(C(C)(C6=C7)CCC(C |
| Physicochemical Information | |
| Melting Point | |
| Boiling Point | |
| Density | |
| Optical Rotation | |
| Reflactive Index | |
| Solubility | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | |
| Chromatograms | |
Mass Spectral Data
Spectroscopic Data
| M.P. | 218 - 219 °C |
| IR (KBr) | 3400-3000 (br), 2929, 1780, 1724, 1645, 1385, 1010 cm-1 |
| 1H-NMR (C5D5N+D2O, 500 MHz) | 0.79 (s, CH3), 1.06 (s, CH3), 1.21 (s, CH3), 1.22 (s, CH3), 1.34 (s, CH3), 1.40 (s, CH3), 1.42 (s, CH3), , 3.02 (brd, J=ca. 12.0 Hz, H-18), 3.35 (dd, J=4.4. 11.2 Hz, H-3), 5.02 (d, J=7.6 Hz, H-1 of GlcUA I), 5.38 (d, J=7.6 Hz, H-1 of GlcUA II), 5.94 (s, H-12) |
| M.P. | 232 - 234 °C |
| 13C-NMR (C5D5N, 22.5MHz) | C-3) 89.0, (11) 198.8, (12) 129.6, (13) 164.3, (18) 44.8, (22) 84.0, (24) 16.4, (29) 28.0, (30) 179.3 GlcUA I (1) 104.6, (2) 83.8, (3) 76.2, (4) 72.2, (5) 77.2, (6) 169.7 GlcUA II (1)106.2, (2) 76.1, (3) 77.2, (4) 72.5, (5) 77.2, (6) 169.8 |
M. Yoshikawa et al., Chem.Pharm.Bull., 41, 1337 (1993).