Koryoginsenoside-R1
From Metabolomics.JP
(Difference between revisions)
(New page: {{Hierarchy|{{PAGENAME}}}} {{Metabolite |SysName=(E)-(3beta,6alpha,12beta)-3,12-Dihydroxy-6-[[6-O-(1-oxo-2-butenyl)-beta-D-glucopyranosyl]oxy]dammar-24-en-20-ylbeta-D-glucopyranoside |Com...) |
|||
| Line 2: | Line 2: | ||
{{Metabolite | {{Metabolite | ||
| − | |SysName=(E)-(3beta,6alpha,12beta)-3,12-Dihydroxy-6- | + | |SysName=(E)-(3beta,6alpha,12beta)-3,12-Dihydroxy-6-((6-O-(1-oxo-2-butenyl)-beta-D-glucopyranosyl)oxy)dammar-24-en-20-yl beta-D-Glucopyranoside |
|Common Name=&&Koryoginsenoside R1&& | |Common Name=&&Koryoginsenoside R1&& | ||
|CAS=171674-97-8 | |CAS=171674-97-8 | ||
Latest revision as of 12:59, 16 February 2010
Upper classes
| IDs and Links | |
|---|---|
| LipidBank | [1] |
| LipidMaps | [2] |
| CAS | 171674-97-8 |
| KEGG | {{{KEGG}}} |
| KNApSAcK | |
| CDX file | |
| MOL file | Koryoginsenoside-R1.mol |
| Koryoginsenoside R1 | |
|---|---|
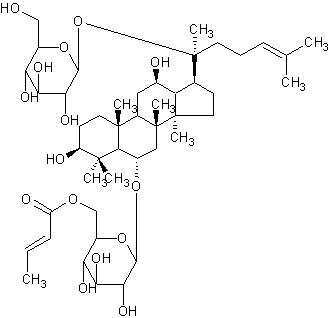
| |
| Structural Information | |
| Systematic Name | (E)-(3beta,6alpha,12beta)-3,12-Dihydroxy-6-((6-O-(1-oxo-2-butenyl)-beta-D-glucopyranosyl)oxy)dammar-24-en-20-yl beta-D-Glucopyranoside |
| Common Name |
|
| Symbol | |
| Formula | C46H76O15 |
| Exact Mass | 868.5184217619999 |
| Average Mass | 869.08664 |
| SMILES | C(C(O)6)(OC(C(C6O)O)COC(=O)C=CC)OC(C54)CC(C3C(CCC( |
| Physicochemical Information | |
| Melting Point | |
| Boiling Point | |
| Density | |
| Optical Rotation | |
| Reflactive Index | |
| Solubility | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | |
| Chromatograms | |
[edit] Spectroscopic Data
| IR (KBr) | 3500-3300 (OH), 2930 (CH), 1710 (conjugated CO, ester), 1640, 1440, 1380, 1300 cm-1 |
| 1H-NMR (C5D5N, 400 MHz) | 0.94 (s, 3xH-30), 1.07 (s, 3xH-19), 1.39 (s, 3xH-18), 1.52 (s, 3xH-29), 1.62 (s, 3xH-26 and 3xH-27), 1.63 (s, 3xH-21), 1.75 (d, J=6.6 Hz, 3xH-4 of But), 2.01 (s, 3xH-28), 3.46 (dd, J=11.5, 4.6 Hz, H-3), 4.11 (m, H-12), 4.37 (dd, J=10.5, 3.6 Hz, H-6), 5.01 (d, J=7.6 Hz, H-1 of Glc I), 5.15 (d, J=7.8 Hz, H-1 of Glc II), 5.27 (t, J=6.7 Hz, H-24), 6.02 (d, J=15.6 Hz, H-2 of But), 7.06 (dq, J=15.6, 7.0 Hz, H-3 of But) |
| 13C-NMR (C5D5N, 100MHz) | C-1) 39.6, (2) 27.9, (3) 78.8, (4) 40.3, (5) 61.5, (6) 80.0, (7) 45.6, (8) 41.4, (9) 50.1, (10) 39.8, (11) 31.0, (12) 70.3, (13) 49.3, (14) 51.5, (15) 31.1, (16) 26.8, (17) 51.8, (18) 17.7, (19) 17.6, (20) 83.4, (21) 22.4, (22) 36.2, (23) 23.3, (24) 126.0, (25) 131.0, (26) 25.8, (27) 17.8, (28) 31.6, (29) 16.5, (30) 17.4 Glc I (1) 106.1, (2) 75.5, (3) 79.2, (4) 71.8, (5) 75.2, (6) 65.1 But (1) 166.6, (2) 123.3, (3) 144.7, (4) 17.6 Glc II (1) 98.3, (2) 75.2, (3) 79.2, (4) 71.6, (5) 78.2, (6) 63.1 |
C.-R. Yang et al., Phytochemistry, 40, 1493 (1995).