Doc:FL63AC
| Line 16: | Line 16: | ||
|- | |- | ||
! Structure | ! Structure | ||
| − | | [[Image:FL63ACNS0001.png| | + | | [[Image:FL63ACNS0001.png|130px]] |
| − | | [[Image:FL63ACNS0002.png| | + | | [[Image:FL63ACNS0002.png|130px]] |
| − | | [[Image:FL63ACNS0004.png| | + | | [[Image:FL63ACNS0004.png|130px]] |
| − | | [[Image:FL63ACNS0003.png| | + | | [[Image:FL63ACNS0003.png|130px]] |
|- | |- | ||
! Name | ! Name | ||
| Line 32: | Line 32: | ||
! 3-Hydroxyl stereo | ! 3-Hydroxyl stereo | ||
| ↑ || ↓ || ↓ || ↑ | | ↑ || ↓ || ↓ || ↑ | ||
| + | |- | ||
| + | ! Afzelechins | ||
| + | | [[FL63AANS0001|(+)-Afzelechin]] | ||
| + | | [[FL63AANS0002|(-)-Epiafzelechin]] | ||
| + | | | ||
| + | | [[FL63AANS0003|ent-Epiafzelechin]] | ||
|- | |- | ||
! Gallocatechins | ! Gallocatechins | ||
| [[FL63AGNS0002|(+)-Gallocatechin]] | | [[FL63AGNS0002|(+)-Gallocatechin]] | ||
| [[FL63AGNS0003|(-)-Epigallocatechin]] | | [[FL63AGNS0003|(-)-Epigallocatechin]] | ||
| − | | [[FL63AGNS0017| | + | | [[FL63AGNS0017|ent-Gallocatechin]] |
| − | | | + | | |
|} | |} | ||
Latest revision as of 11:58, 19 August 2010
|
Catechins refer to a subgroup of flavan 3-ol derivatives (FL63AC). The two chiral center at C2 and C3 of the flavan 3-ols produces 4 isomers, and (+)-Catechin and its stereoisomer (-)-Epicatechin are naturally abundant. Less abundant are (-)-Catechin and (+)-Epicatechin.
|
いわゆるカテキン類とは、Flavan 3-olの下にあるグループを指します。C2, C3位にある不斉炭素によって4つの異性体が作られますが、自然界に豊富なのは (+)-カテキン とその立体異性体である (-)-エピカテキン です。 (-)-カテキン と (+)-エピカテキン は自然界にあまりみられません。
|
| Structure | 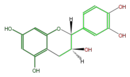
|
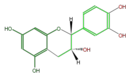
|
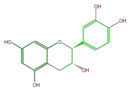
|
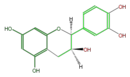
|
|---|---|---|---|---|
| Name | (+)-Catechin or D-Catechin |
(-)-Epicatechin or L-Epicatechin |
ent-Catechin or (-)-Catechin |
ent-Epicatechin or (+)-Epicatechin |
| B-ring stereo | ↓ | ↓ | ↑ | ↑ |
| 3-Hydroxyl stereo | ↑ | ↓ | ↓ | ↑ |
| Afzelechins | (+)-Afzelechin | (-)-Epiafzelechin | ent-Epiafzelechin | |
| Gallocatechins | (+)-Gallocatechin | (-)-Epigallocatechin | ent-Gallocatechin |
|
Among the stereoisomers, the bioavailability in human follows the order: |
立体異性体の中で、ヒトにおける利用活性は以下の順番になります。 |
| (-)-epicatechin | > | (+)-catechin | = | (+)-epicatechin | > | (-)-catechin |
|
The circulation level of (-)-epicatechin is 6 times higher than that of (-)-catechin. Naturally abundant species are more bioavailable than less abundant ones. |
(-)-エピカテキンが体内に入る効率は(-)-カテキンの6倍にもなり、天然に多く産する分子種が多く吸収されることがわかります。 |