Category:PK
m (→{{Bilingual|構造|Structure}}) |
m (→{{Bilingual|構造|Structure}}) |
||
| Line 10: | Line 10: | ||
{{Twocolumn| | {{Twocolumn| | ||
Polyketides are natural products with multiple ketone structures and synthesized from acetyl CoA. The synthetic pathway is called Acetate-Malonate Pathway. | Polyketides are natural products with multiple ketone structures and synthesized from acetyl CoA. The synthetic pathway is called Acetate-Malonate Pathway. | ||
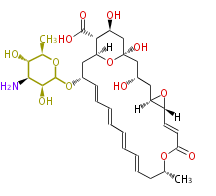
| − | Well known products include | + | Well known products include erythromycin (antibiotic), melanin (pigment), and aflatoxin (toxin). |
| | | | ||
ポリケチドはケトン構造を複数持つ天然物のカテゴリーで、アセチルCoAから作られます。その生合成ステップは、酢酸-マロン酸経路と呼ばれています。代表例は抗生物質のエリスロマイシン、色素のメラニン、毒素のアフラトキシンです。 | ポリケチドはケトン構造を複数持つ天然物のカテゴリーで、アセチルCoAから作られます。その生合成ステップは、酢酸-マロン酸経路と呼ばれています。代表例は抗生物質のエリスロマイシン、色素のメラニン、毒素のアフラトキシンです。 | ||
Revision as of 12:05, 18 December 2012
| Polyketide Top | Species List | UniRef90 Class | UniRef50 Class | Gene Class | Domains (by CDD) |
Domains (by MAPSI) |
Polyketide
|
Overview
Structure
Polyketides are natural products with multiple ketone structures and synthesized from acetyl CoA. The synthetic pathway is called Acetate-Malonate Pathway. Well known products include erythromycin (antibiotic), melanin (pigment), and aflatoxin (toxin).
Acetate-Malonate Pathway
Acetyl CoA from glycolysis and its carboxylated form (malonyl CoA) are polymerized to form polyketone as in the fatty acid synthesis without reduction of carbonyl groups. Methylene moieties between ketones are highly reactive and easily Aldol- or Claisen-condensed to generate aromatic rings.
Elongation Mechanism
Polyketides are synthesized through the polymerization of acetyl units (β-ketomethylene) as in fatty acid biosynthesis. Typical starter units are short-chain fatty acids (e.g. acetyl-CoA or propionyl-CoA), on to which extender units (e.g. malonyl-CoA or methylmalonyl-CoA) are repeatedly polymerized. The key reactions for the chain extension are:
- Claisen condensation by β-ketoacyl synthase (KS)
- an acyltransferase (AT), and
- an acyl carrier protein (ACP).
After elongation, β-ketone is reduced. In fatty acid biosynthesis, the chain is fully reduced by the following three steps:
- Reduction to an alcohol by ketoreductase (KR),
- Dehydration to the conjugated ester by dehydratase (DH), and
- Reduction of the double bond by enoyl reductase (ER).
Finally, the chain is terminated by a thioesterase (TE) activity and allows Claisen cyclization (CYC).
Polyketide Synthase (PKS)
Distribution
PKS members are found in bacteria, fungi, plants, slime mold[1], Alveolata[2], and animals [3][4].
| Species | Actinomycetes | Cyanobacteria | γ-Proteobacteria | Fungi | Alveolata |
|---|---|---|---|---|---|
| Type-I PKS | Ο | Ο | Ο | Ο | Ο |
| Type-II PKS | Ο | Χ | Χ | Χ | Χ |
| NRPS | Ο | Ο | Ο | Ο | Χ |
| deoxysugar | Ο | Χ | Χ | Χ | Χ |
| Terpene | Δ | Χ | Χ | Ο | Χ |
- References
- ↑ Zucko J, Skunca N, Curk T, Zupan B, Long PF et al (2007) "Polyketide synthase genes and the natural products potential of Dictyostelium discoideum" Bioinformatics 23:2543-49
- ↑ Zhu G, LaGier MJ, Stejskal F, Millership JJ, Cai X et al (2002) "Cryptosporidium parvum: the first protist known to encode a putative polyketide synthase" Gene 298:79-89
- ↑ Castoe TA, Stephens T, Noonan BP, Calestani C (2007) "A novel group of type I polyketide synthases (PKS) in animals and the complex phylogenomics of PKSs" Gene 392:47-58
- ↑ Calestani C, Rast JP, Davidson EH (2003) "Isolation of pigment cell specific genes in the sea urchin embryo by differential macroarray screening" Development 130:4587-96
Type
There are three types of PKSs known to date.
Type I
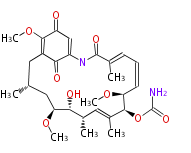
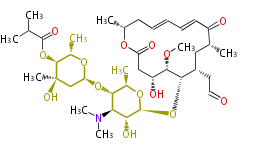
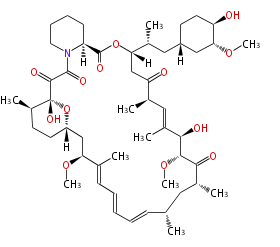
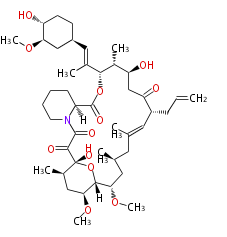
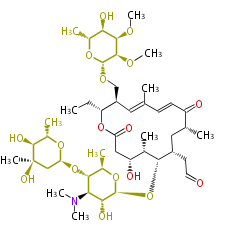
Multiple domains per protein (e.g. Erythromycin biosynthesis [1])
- Bacterial type I is modular, i.e., each domain (or module) catalyses a specific transformation.
- Non-reducing (NR) type produces aromatic polyketides
- partially reducing (PR) type
- highly reducing (HR) type
- (cf. fully reducing cases are fatty acid synthases, FAS)
- NR-PKS
- orsellinic acid
- PR-PKS
- 6-methyl salicylic acid (MSA)
Type II
single domain per protein
- Three proteins (KSα, KSβ, ACP) are repeatedly used for carbon chain elongation, and the chain length is determined by another protein, CLF.
- In bacteria, products are aromatic (e.g. chiorotetracycline, pradimicin).
Type III
Chalcone synthase-like in plants
- Discovered in plants, but later found in bacteria[2]
Unusual Type
- Bacterial but iterative type I PKS for aromatic polyketide
- AviM for orsellinic acid biosynthesis (Streptomyces viridochromogens Tu57)[3]
- CalO5 for calicheamicin biosyntehsis (Micromonospora echinospora ssp. calichenisis)[4]
- NesB for neocarzinostatin biosynthesis (?)[5]
- Type I PKS that lacks the cognate AT domain
- lnmIJ for leinamycin biosynthesis (Streptomyces atroolivaceus S-140)[6]
- PedF for pederin biosynthesis (symbiont bacterium of Paederus beetles)[7]
- Type II PKS that act non-iteratively and use acyl CoA as substrates directly
- NonPQU and NonJK (Streptomyces griseus)[8]
Unusual structures
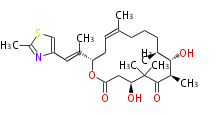
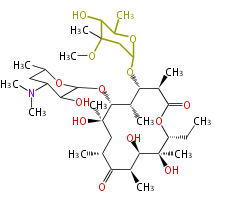
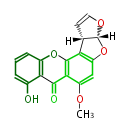
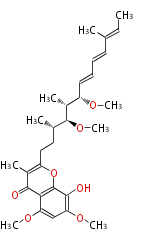
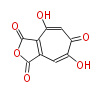
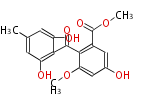
| Phoma | zaragozic acid, phomoidoride | Streptomyces | yatakemycin, leinamycin, saframycin, neocarzinostatin, staurosporin, FR182877 | Other bacteria | PKS-NRPS hybrid type
Curacin A (Lyngbya), Shiphonazole (Herpetosiphon), Jamaicamide A (Lyngbya), Cylindrospermopsin (Cylindrospermopsis) |
|---|
Non-ribosomal peptide synthase (NRPS)
Coupling with PKS and NRPS
- vancomycin ()
- leinamycin (Curr opin chem biol 7:285, 2003)
- pseurotin (chem bio chem 8:1736-1743, 2007)
- curacin (curr opin chem biol 13:216, 2009)
- epothilone
- rapamycin
Decoration
deoxysugars
deoxygenation, c-methylation, amination, n-methylation, ketosugar,
- References
- ↑ (2001) Nat Prod Rep 18:380
- ↑ Moore BS, Hopke JN (2001) Discovery of a new bacterial polyketide biosynthetic pathway Chembiochem 2:35-8
- ↑ Gaisser S, Trefzer A, Stockert S, Kirschning A, Bechthold A (1997) Cloning of an avilamycin biosynthetic gene cluster from Streptomyces viridochromogenes Tu57. J Bacteriol 179:6271-8
- ↑ Whitwam RE, Ahlert J, Holman TR, Ruppen M, Thorson JS (2000) The gene calC encodes for a non-heme iron metalloprotein responsible for calicheamicin self-resistance in Micromonospora. J Am Chem Soc 122:1556-7
- ↑ Zazopoulos E, Huang K, Staffa A, Liu W, Bachmann BO, Nonaka K, Ahlert J, Thorson JS, Shen B, Farnet CM (2003) A genomics-guided approach for discovering and expressing cryptic metabolicpathways Nat Biotechnol epub.
- ↑ Cheng Y-Q, Tang G-L, Shen B (2003) Type I polyketide synthase requiring a discrete acyltransferase for polyketide biosynthesis Proc Natl Acad Sci U S A 100: in press
- ↑ Piel J (2002) A polyketide synthase-peptide synthetase gene cluster from an uncultured bacterial symbiont of Paederus beetles Proc Natl Acad Sci U S A 98:14808-13
- ↑ Kwon HJ, Smith WC, Scharon AJ, Hwang SH, Kurth MJ, Shen B (2002) C-O bond formation by polyketide synthases Science 297(5585):1327-30