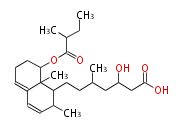Category:PK
m |
|||
| Line 7: | Line 7: | ||
{{Twocolumn| | {{Twocolumn| | ||
| − | Polyketides are synthesized through the polymerization of acetyl units (β-ketomethylene chain). | + | Polyketides are synthesized through the polymerization of acetyl units (β-ketomethylene) as in fatty acid biosynthesis. Typical starter units are short-chain fatty acids (e.g. acetyl-CoA or propionyl-CoA), on to which extender units (e.g. malonyl-CoA or methylmalonyl-CoA) are repeatedly polymerized. |
| − | The key reactions for chain | + | The key reactions for the chain extension are: |
* Claisen condensation by β-ketoacyl synthase ('''KS''') | * Claisen condensation by β-ketoacyl synthase ('''KS''') | ||
* an acyltransferase ('''AT'''), and | * an acyltransferase ('''AT'''), and | ||
* an acyl carrier protein ('''ACP'''). | * an acyl carrier protein ('''ACP'''). | ||
| − | After elongation, | + | |
| − | * | + | After elongation, β-ketone is reduced. In fatty acid biosynthesis, the chain is fully reduced by the following three steps: |
| − | * | + | * Reduction to an alcohol by ketoreductase ('''KR'''), |
| − | * | + | * Dehydration to the conjugated ester by dehydratase ('''DH'''), and |
| + | * Reduction of the double bond by enoyl reductase ('''ER'''). | ||
| + | In polyketide synthase, the reduction is patial. | ||
| + | |||
Finally, the chain is terminated by a thioesterase ('''TE''') activity and | Finally, the chain is terminated by a thioesterase ('''TE''') activity and | ||
| − | allows cyclization ( | + | allows Claisen cyclization ('''CYC'''). |
| | | | ||
ポリケチドはアセチル単位 (β-ケトメチレン鎖) の重合によって作られます。 | ポリケチドはアセチル単位 (β-ケトメチレン鎖) の重合によって作られます。 | ||
| Line 24: | Line 27: | ||
* アシル基転移酵素 ('''AT''') による伸長と、それを支える | * アシル基転移酵素 ('''AT''') による伸長と、それを支える | ||
* アシル輸送タンパク質 ('''ACP''') | * アシル輸送タンパク質 ('''ACP''') | ||
| − | + | です。 | |
| + | |||
| + | また、伸張後に重要な反応は | ||
* ケト還元酵素 ('''KR''') によるアルコールへの還元 | * ケト還元酵素 ('''KR''') によるアルコールへの還元 | ||
* 脱水酵素 ('''DH''') による共役エステルからの脱水 | * 脱水酵素 ('''DH''') による共役エステルからの脱水 | ||
* エノイル還元酵素 ('''ER''') による二重結合の還元 | * エノイル還元酵素 ('''ER''') による二重結合の還元 | ||
| − | + | です。 | |
| − | + | ||
| + | 最後に、チオエステル分解酵素 ('''TE''') によって伸張が止まり、ラクトン化 (閉環) します。 | ||
| + | }} | ||
<center> | <center> | ||
| Line 69: | Line 75: | ||
{| class="wikitable" | {| class="wikitable" | ||
| − | + | !colspan="4"| Linear Chain and Related () | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | !colspan="4"| Linear Chain and Related | + | |
|- | |- | ||
| | | | ||
{| class="collapsible collapsed" style="width:150px" | {| class="collapsible collapsed" style="width:150px" | ||
| − | ! | + | ! Straight (LS) |
|- | |- | ||
| linear | | linear | ||
| Line 87: | Line 85: | ||
| | | | ||
{| class="collapsible collapsed" style="width:150px" | {| class="collapsible collapsed" style="width:150px" | ||
| − | ! Polyether | + | ! Polyether (LE) |
|- | |- | ||
| nigericin | | nigericin | ||
|- | |- | ||
| monensin | | monensin | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
|- | |- | ||
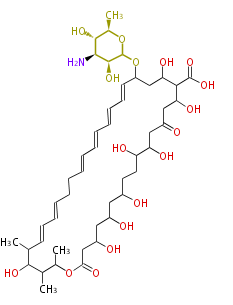
| − | | | + | | okadaic acid |
|- | |- | ||
| − | | | + | | ciguatoxin, brevetoxin |
| + | |- | ||
| + | | halichondrin | ||
| + | |- | ||
| + | | zaragozic acid | ||
|} | |} | ||
| + | | Acetogenins (LA) | ||
| + | | | ||
|- | |- | ||
| − | !colspan="4"| Aromatic and Related | + | !colspan="4"| Aromatic and Diels-Alder Related (most often by iterative type II) |
|- | |- | ||
| | | | ||
{| class="collapsible collapsed" style="width:150px" | {| class="collapsible collapsed" style="width:150px" | ||
| − | ! | + | ! Monocyclic (A1) |
|- | |- | ||
| − | | | + | | Salicylic acid |
|- | |- | ||
| orsellinic acid | | orsellinic acid | ||
| Line 119: | Line 118: | ||
| | | | ||
{| class="collapsible collapsed" | {| class="collapsible collapsed" | ||
| − | ! | + | ! Bicyclic (A2) |
|- | |- | ||
| lovastatin | | lovastatin | ||
|- | |- | ||
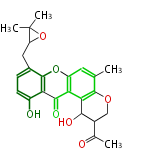
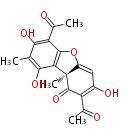
| − | | aflatoxins | + | | aflatoxins<ref></ref> |
|} | |} | ||
| | | | ||
{| class="collapsible collapsed" | {| class="collapsible collapsed" | ||
| − | ! | + | ! Tricyclic (A3) |
|- | |- | ||
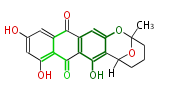
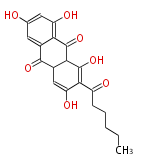
| emodin | | emodin | ||
| Line 137: | Line 136: | ||
| | | | ||
{| class="collapsible collapsed" style="width:150px" | {| class="collapsible collapsed" style="width:150px" | ||
| − | ! | + | ! Tetracyclic (A4) |
|- | |- | ||
| Linear type | | Linear type | ||
| Line 146: | Line 145: | ||
!colspan="4"| Macrolides (most often by non-iterative type I) | !colspan="4"| Macrolides (most often by non-iterative type I) | ||
|- | |- | ||
| − | |style="width:150px"| Polyene | + | |style="width:150px"| |
| − | + | {| class="collapsible collapsed" style="width:150px" | |
| − | + | ! Polyene (MN) | |
| − | + | |- | |
| − | |style="width:150px"| | + | | Manumycin |
| − | + | |- | |
| − | + | | Nystatin | |
| − | + | |- | |
| − | + | | Amphotericin | |
| − | + | |} | |
| − | + | |style="width:150px"| | |
| − | + | {| class="collapsible collapsed" style="width:150px" | |
| − | + | ! Cyclic Imines (MI) | |
| − | + | |- | |
| − | + | | Spirolide | |
| − | + | |- | |
| − | + | | Pinnatoxin | |
| − | + | |} | |
| − | + | |style="width:150px"| | |
| + | {| class="collapsible collapsed" style="width:150px" | ||
| + | ! Ansamacrolide (MA) | ||
| + | |- | ||
| + | | Rifamycin | ||
| + | |- | ||
| + | | Ansamycin | ||
| + | |} | ||
| + | |style="width:150px"| | ||
| + | {| class="collapsible collapsed" style="width:150px" | ||
| + | ! Polyether (ME) | ||
| + | |- | ||
| + | | Nonactin, Nactin | ||
| + | |} | ||
| + | |- | ||
| + | |style="width:150px"| | ||
| + | {| class="collapsible collapsed" style="width:150px" | ||
| + | ! 12-membered (M2) | ||
| + | |- | ||
| | | | ||
| + | |} | ||
| + | |style="width:150px"| | ||
| + | {| class="collapsible collapsed" style="width:150px" | ||
| + | ! 14-membered (M4) | ||
| + | |- | ||
| + | | Colletodiol | ||
| + | |- | ||
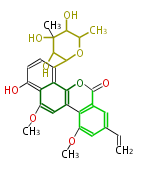
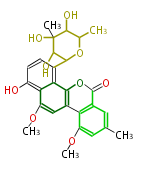
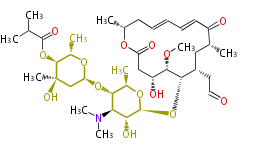
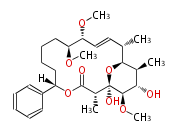
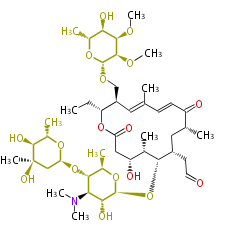
| + | | Erythromycin<ref>6-deoxy sugars (L-cladinose and D-desosamine) are attached.</ref> | ||
| + | |- | ||
| + | | Fluvirucin | ||
| + | |- | ||
| + | | Zearalenone | ||
| + | |} | ||
| + | |style="width:150px"| | ||
| + | {| class="collapsible collapsed" style="width:150px" | ||
| + | ! 16-membered (M6) | ||
| + | |- | ||
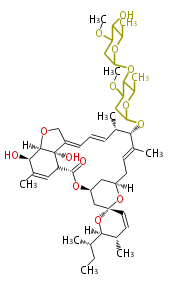
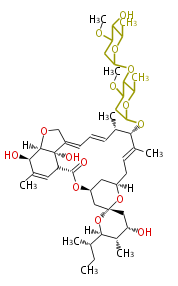
| + | | Avermectin | ||
| + | |- | ||
| + | | Bafilomycin | ||
| + | |- | ||
| + | | Oligomycin | ||
| + | |- | ||
| + | | Tylosin | ||
| + | |} | ||
| + | |style="width:150px"| | ||
| + | {| class="collapsible collapsed" style="width:150px" | ||
| + | ! More (MM) | ||
| + | |- | ||
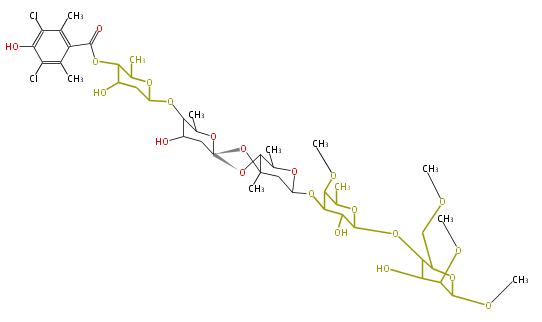
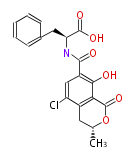
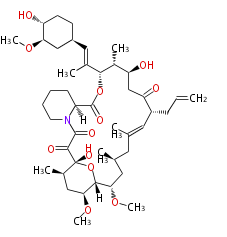
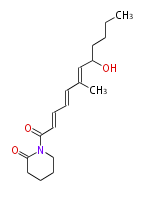
| + | | Bryostatin (26)<ref>It lacks AT domain as in mupirocin, leinamycin<ref>Nguyen T, Ishida K, Jenke-Kodama H, Dittmann E, Gurgui C, Hochmuth T, Taudien S, Platzer M, Hertweck C, Piel J (2008) "Exploiting the mosaic structure of trans-acyltransferase polyketide synthases for natural product discovery and pathway dissection" ''Nat Biotechnol'' 26:225 - 233 PMID 18223641</ref> | ||
| + | |- | ||
| + | | Milbemycin (20) | ||
| + | |- | ||
| + | | Myxovirescin (28)<ref>http://www.indiana.edu/~drwchem/pdfs/50.pdf</ref> | ||
| + | |- | ||
| + | | Natamycin (26)<ref>=Pimaricin</ref> | ||
| + | |- | ||
| + | | Tacrolimus (23) | ||
| + | |} | ||
|} | |} | ||
Revision as of 00:28, 18 December 2010
Polyketide (ポリケチド)
|
Class Overview
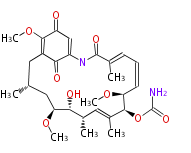
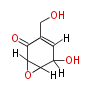
Polyketides are synthesized through the polymerization of acetyl units (β-ketomethylene) as in fatty acid biosynthesis. Typical starter units are short-chain fatty acids (e.g. acetyl-CoA or propionyl-CoA), on to which extender units (e.g. malonyl-CoA or methylmalonyl-CoA) are repeatedly polymerized. The key reactions for the chain extension are:
- Claisen condensation by β-ketoacyl synthase (KS)
- an acyltransferase (AT), and
- an acyl carrier protein (ACP).
- Reduction to an alcohol by ketoreductase (KR),
- Dehydration to the conjugated ester by dehydratase (DH), and
- Reduction of the double bond by enoyl reductase (ER).
| 1st Class | ||
|---|---|---|
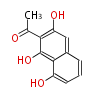
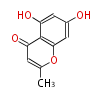
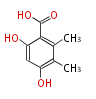
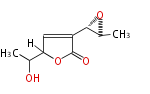
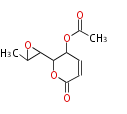
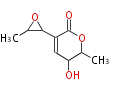
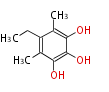
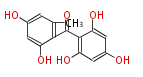
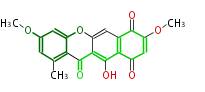
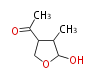
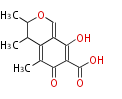
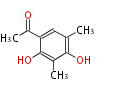
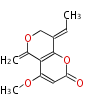
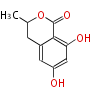
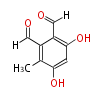
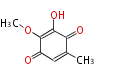
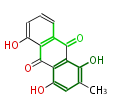
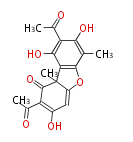
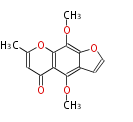
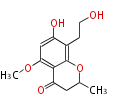
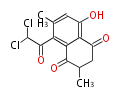
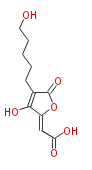
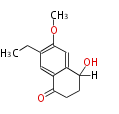
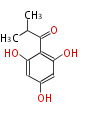
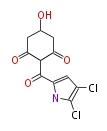
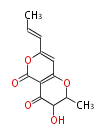
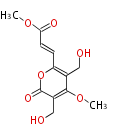
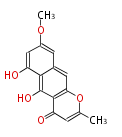
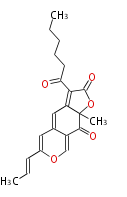
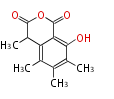
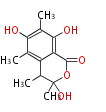
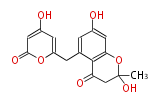
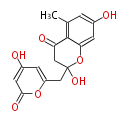
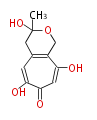
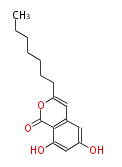
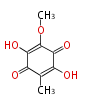
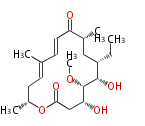
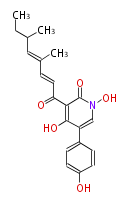
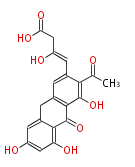
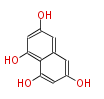
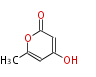
| PK4: Four C2 Units orsellinic acid, 6-methylsalicylic acid, triacetic acid lactone, asperlin, usnic acid, methylphloracetophenone, penicillic acid, patulin |
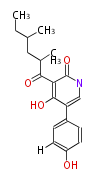
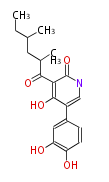
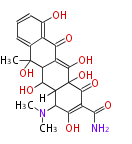
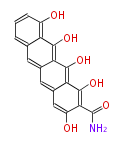
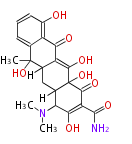
PK5: Five C2 Units citrinin, aflatoxin, augenone, sepedonin, stipitatonic acid |
PK6: Six C2 Units plumbagin, 7-methyljuglone, juglone, variotin |
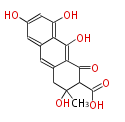
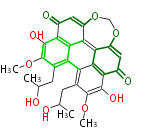
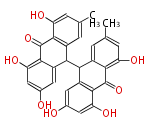
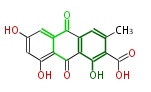
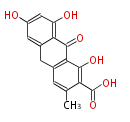
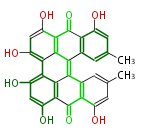
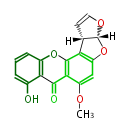
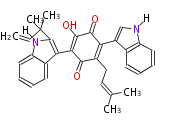
| PK7: Seven and eight C2 Units Anthraquinone rings |
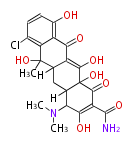
PK9: Nine C2 Units Tetracyclines | |
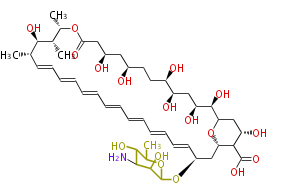
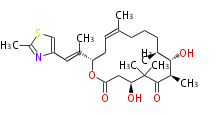
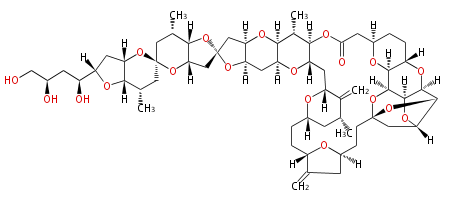
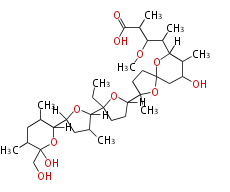
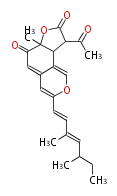
| Linear Chain and Related () | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
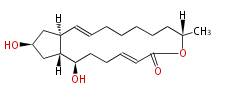
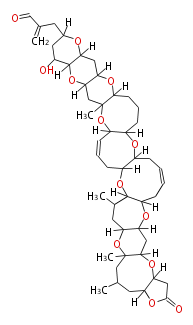
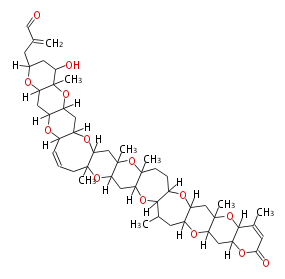
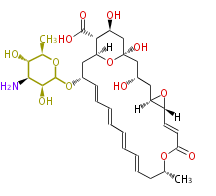
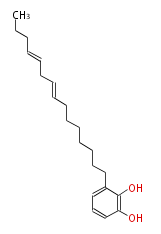
Acetogenins (LA) | |||||||||||||||||||
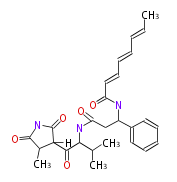
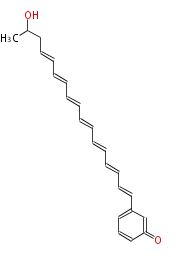
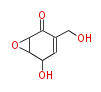
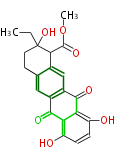
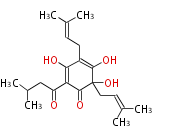
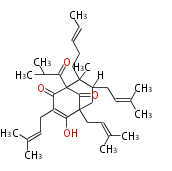
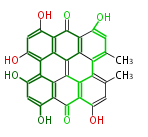
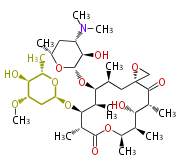
| Aromatic and Diels-Alder Related (most often by iterative type II) | |||||||||||||||||||||
|
|
|
| ||||||||||||||||||
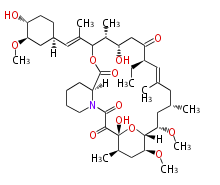
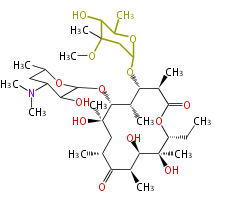
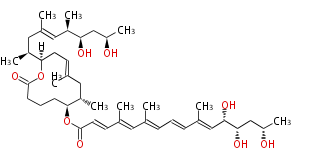
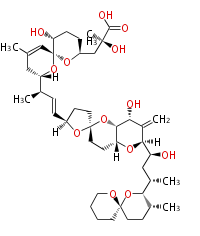
| Macrolides (most often by non-iterative type I) | |||||||||||||||||||||
|
|
|
| ||||||||||||||||||
|
|
|
| ||||||||||||||||||
Polyketide Synthase (PKS)
| species | Actinomycetes | Cyanobacteria | γ-Proteobacteria | Fungi | Dinoflagellates |
|---|---|---|---|---|---|
| Type-I PKS | Ο | Ο | Ο | Χ | Ο |
| Type-II PKS | Ο | Χ | Χ | Ο | Χ |
| NRPS | Ο | Ο | Ο | Ο | Χ |
| deoxysugar | Ο | Χ | Χ | Χ | Χ |
| Terpene | Δ | Χ | Χ | Ο | Χ |
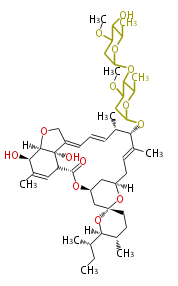
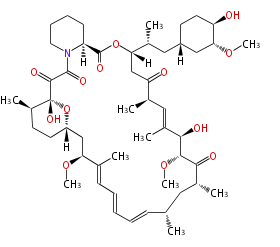
Type I PKS (non-iterative)
- Multi catalytic domains exist in a single protein
- Chain length is determined by the number of catalytic domains.
- Products are non-aromatic and have larger masses.
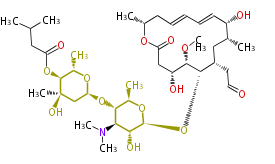
Ref. Erythromycin biosynthesis in Nat Prod Rep 18, 380 (2001)
Type II PKS (iterative)
- Three proteins (KSα, KSβ, ACP) are repeatedly used for carbon chain elongation.
- Chain length is determined by another protein, CLF.
- In bacteria, products are aromatic (e.g. chiorotetracycline, pradimicin).
- In fungi, products are both non-aromatic and aromatic.
Non-ribosomal peptide synthase (NRPS)
Coupling with PKS and NRPS
- vancomycin ()
- leinamycin (Curr opin chem biol 7:285, 2003)
- pseurotin (chem bio chem 8:1736-1743, 2007)
- curacin (curr opin chem biol 13:216, 2009)
- epothilone
- rapamycin
PKS in Fungi
- both aromatic and non-aromatic compounds are generated by iterative PKS
- methyl branch is transferred from methionine, not methylmalonyl CoA
Ref. Dewick, PM Medicinal Natural Products (2009)
Decoration
deoxysugars
deoxygenation, c-methylation, amination, n-methylation, ketosugar,
Unusual structures
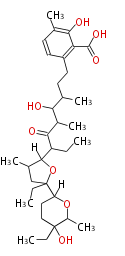
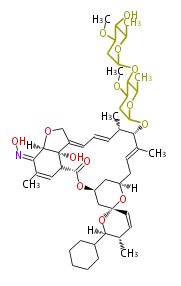
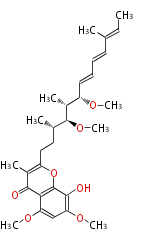
| Phoma | zaragozic acid, phomoidoride | Streptomyces | yatakemycin, leinamycin, saframycin, neocarzinostatin, staurosporin, FR182877 | Other bacteria | PKS-NRPS hybrid type
Curacin A (Lyngbya), Shiphonazole (Herpetosiphon), Jamaicamide A (Lyngbya), Cylindrospermopsin (Cylindrospermopsis) |
|---|
Cite error:
<ref> tags exist, but no <references/> tag was found