Category:FLN
From Metabolomics.JP
(Difference between revisions)
| Line 1: | Line 1: | ||
| − | + | {{Huge|Neoflavonoid}} | |
| + | |||
| + | {{Hierarchy|{{PAGENAME}}}} | ||
| + | |||
| + | =={{Bilingual|ネオフラボノイドの概要|Overview}}== | ||
| + | |||
| + | {| class="wikitable" border="1" cellspacing="0" | ||
| + | |- | ||
| + | ! colspan="6"|2nd Class | ||
| + | |- | ||
| + | |FLNA||[[:Category:FLNA|4-Arylcoumarin]]<br>[[Image:Flna.png|72px]] | ||
| + | |FLNB||[[:Category:FLNB|4-Arylchroman]]<br>[[Image:Flnb.png|72px]] | ||
| + | |FLNC||[[:Category:FLNC|Dalbergiquinol]]<br>[[Image:Flnc.png|72px]] | ||
| + | |- | ||
| + | |FLND||[[:Category:FLND|Dalbergione]]<br>[[Image:Flnd.png|65px]] | ||
| + | |FLNE||[[:Category:FLNE|Neoflavene]]<br>[[Image:Flne.png|50px]] | ||
| + | |FLNF||[[:Category:FLNF|Coumarinic acid]]<br>[[Image:Flnf.png|72px]] | ||
| + | |} | ||
| + | |||
| + | =={{Bilingual|データベース統計|Database statistics}}==<!--- see FL1---> | ||
| + | {{#def:FLN|{{#SearchTitle:FLN|}}}} | ||
| + | {{#def:rFLN|{{#SearchLine:&&FLN|Reference}}}} | ||
| + | {{#graph:pie|size=350x200;title=Registered Molecules | ||
| + | ({{#count:{{#var:FLN}}}} total);legend=0.01x0.2;label=; | ||
| + | FLNA={{#count:{{#choose:{{#var:FLN}}|FLNA}}}}; | ||
| + | FLNB={{#count:{{#choose:{{#var:FLN}}|FLNB}}}}; | ||
| + | FLNC={{#count:{{#choose:{{#var:FLN}}|FLNC}}}}; | ||
| + | FLND={{#count:{{#choose:{{#var:FLN}}|FLND}}}}; | ||
| + | FLNE={{#count:{{#choose:{{#var:FLN}}|FLNE}}}}; | ||
| + | FLNF={{#count:{{#choose:{{#var:FLN}}|FLNF}}}}; | ||
| + | }} | ||
| + | {{#graph:pie|size=350x200;title=Metabolite-Species Data ({{#countLine:&&FLN|Reference}} total);legend=0.01x0.2;label=; | ||
| + | FLNA={{#count:{{#choose:{{#var:rFLN}}|&&FLNA}}}}; | ||
| + | FLNB={{#count:{{#choose:{{#var:rFLN}}|&&FLNB}}}}; | ||
| + | FLNC={{#count:{{#choose:{{#var:rFLN}}|&&FLNC}}}}; | ||
| + | FLND={{#count:{{#choose:{{#var:rFLN}}|&&FLND}}}}; | ||
| + | FLNE={{#count:{{#choose:{{#var:rFLN}}|&&FLNE}}}}; | ||
| + | FLNF={{#count:{{#choose:{{#var:rFLN}}|&&FLNF}}}}; | ||
| + | }} | ||
| + | {{FL_pie_chart|FLN}} | ||
| + | [[Category:FL]] | ||
| + | |||
| + | <!--- Class Information (Do not delete. It is used by Template:Hierarchy.) | ||
| + | &&FLN&&Neoflavonoid&& | ||
| + | &&FLNA&&4-Arylcoumarin&& | ||
| + | &&FLNA1C&&7,3',4'-Trihydroxy-4-phenylcoumarin and O-methyl derivatives&& | ||
| + | &&FLNA1CNS&&Simple substitution&& | ||
| + | &&FLNA29&&6,7,(3'),(5')-Trihydroxy-4-phenylcoumarin and O-methyl derivatives&& | ||
| + | &&FLNA29NS&&Simple substitution&& | ||
| + | &&FLNA2A&&6,7,4'-Trihydroxy-4-phenylcoumarin and O-methyl derivatives&& | ||
| + | &&FLNA2ANS&&Simple substitution&& | ||
| + | &&FLNA2C&&6,7,3',4'-Tetrahydroxy-4-phenylcoumarin and O-methyl derivatives&& | ||
| + | &&FLNA2CNS&&Simple substitution&& | ||
| + | &&FLNA49&&6,7,8,(3'),(5')-Trihydroxy-4-phenylcoumarin and O-methyl derivatives&& | ||
| + | &&FLNA49NS&&Simple substitution&& | ||
| + | &&FLNAA9&&5,7,(3'),(5')-Trihydroxy-4-phenylcoumarin and O-methyl derivatives&& | ||
| + | &&FLNAA9GS&&O-Glycoside&& | ||
| + | &&FLNAA9NF&&Furanoflavonoid&& | ||
| + | &&FLNAA9NI&&Non-cyclic prenyl substituted&& | ||
| + | &&FLNAA9NP&&Pyranoflavonoid&& | ||
| + | &&FLNAA9NS&&Simple substitution&& | ||
| + | &&FLNAAB&&5,7-Dihydroxy-4'-methoxy-4-phenylcoumarin&& | ||
| + | &&FLNAABNS&&Simple substitution&& | ||
| + | &&FLNAAC&&5,7,3',4'-Tetrahydroxy-4-phenylcoumarin&& | ||
| + | &&FLNAACGS&&O-Glycoside&& | ||
| + | &&FLNAAL&&5,7,2',(3'),4',(5'),(6')-Hydroxy-4-phenylcoumarin&& | ||
| + | &&FLNAALGS&&O-Glycoside&& | ||
| + | &&FLNAALNM&&C-Methyl or C2/C3 substituted&& | ||
| + | &&FLNABE&&7,3'-Dihydroxy-5,4'-dimethoxy-4-phenylcoumarin&& | ||
| + | &&FLNABENM&&C-Methyl or C2/C3 substituted&& | ||
| + | &&FLNABENS&&Simple substitution&& | ||
| + | &&FLNACA&&5,4'-Dihydroxy-7-methoxy-4-phenylcoumarin&& | ||
| + | &&FLNACAGS&&O-Glycoside&& | ||
| + | &&FLNACB&&5-Hydroxy-7,4'-dimethoxy-4-phenylcoumarin&& | ||
| + | &&FLNACBGS&&O-Glycoside&& | ||
| + | &&FLNACC&&5,3',4'-Trihydroxy-7-methoxy-4-phenylcoumarin&& | ||
| + | &&FLNACCGS&&O-Glycoside&& | ||
| + | &&FLNACCNS&&Simple substitution&& | ||
| + | &&FLNACE&&5,3'-Dihydroxy-7,4'-dimethoxy-4-phenylcoumarin&& | ||
| + | &&FLNACENS&&Simple substitution&& | ||
| + | &&FLNADA&&4'-Hydroxy-5,7-dimethoxy-4-phenylcoumarin&& | ||
| + | &&FLNADANS&&Simple substitution&& | ||
| + | &&FLNADB&&5,7,4'-Trimethoxy-4-phenylcoumarin&& | ||
| + | &&FLNADBNS&&Simple substitution&& | ||
| + | &&FLNADC&&3',4'-Dihydroxy-5,7-dimethoxy-4-phenylcoumarin&& | ||
| + | &&FLNADCNS&&Simple substitution&& | ||
| + | &&FLNADE&&3'-Hydroxy-5,7,4'-trimethoxy-4-phenylcoumarin&& | ||
| + | &&FLNADENS&&Simple substitution&& | ||
| + | &&FLNAFA&&5,7,8,4'-Tetrahydroxy-4-phenylcoumarin and O-methyl derivatives&& | ||
| + | &&FLNAFANS&&Simple substitution&& | ||
| + | &&FLNAFC&&5,7,8,3',4'-Pentahydroxy-4-phenylcoumarin and O-methyl derivatives&& | ||
| + | &&FLNAFCNS&&Simple substitution&& | ||
| + | &&FLNAQU&&4-Arylcoumarin quinone&& | ||
| + | &&FLNAQUNI&&Non-cyclic prenyl substituted&& | ||
| + | &&FLNARN&&5-O-2'-Cycled 4-aryocoumarin and O-methyl derivatives&& | ||
| + | &&FLNARNNS&&Simple substitution&& | ||
| + | &&FLNB&&4-Arylchroman&& | ||
| + | &&FLNB2C&&Brazilin and O-methyl derivatives&& | ||
| + | &&FLNB2CNS&&Simple substitution&& | ||
| + | &&FLNB3C&&Haematoxylin and O-methyl derivatives&& | ||
| + | &&FLNB3CNS&&Simple substitution&& | ||
| + | &&FLNC&&Dalbergiquinol&& | ||
| + | &&FLNC28&&2,4,5,2',(3'),(5'),(6')-Hydroxydalbergiquinol and O-methyl derivatives&& | ||
| + | &&FLNC28NS&&Simple substitution&& | ||
| + | &&FLNC29&&2,4,5,(3'),(5')-Hydroxydalbergiquinol and O-methyl derivatives&& | ||
| + | &&FLNC29NS&&Simple substitution&& | ||
| + | &&FLNC49&&2,3,4,5,(3'),(5')-Hydroxydalbergiquinol and O-methyl derivatives&& | ||
| + | &&FLNC49NS&&Simple substitution&& | ||
| + | &&FLNC4A&&2,3,4,5,4'-Pentahydroxydalbergiquinol and O-methyl derivatives&& | ||
| + | &&FLNC4ANS&&Simple substitution&& | ||
| + | &&FLND&&Dalbergione&& | ||
| + | &&FLND29&&4,(3'),(5')-Hydroxydalbergione and O-methyl derivatives&& | ||
| + | &&FLND29NS&&Simple substitution&& | ||
| + | &&FLND2A&&4,4'-Hydroxydalbergione and O-methyl derivatives&& | ||
| + | &&FLND2ANS&&Simple substitution&& | ||
| + | &&FLND2C&&4,3',4'-Hydroxydalbergione and O-methyl derivatives&& | ||
| + | &&FLND2CNS&&Simple substitution&& | ||
| + | &&FLND49&&3,4,(3'),(5')-Hydroxydalbergione and O-methyl derivatives&& | ||
| + | &&FLND49NS&&Simple substitution&& | ||
| + | &&FLND4A&&3,4,4'-Hydroxydalbergione and O-methyl derivatives&& | ||
| + | &&FLND4ANS&&Simple substitution&& | ||
| + | &&FLNE&&Neoflavene&& | ||
| + | &&FLNE29&&6,7,(3),(5)-Hydroxyneoflavene and O-methyl derivatives&& | ||
| + | &&FLNE29NS&&Simple substitution&& | ||
| + | &&FLNE49&&6,7,8,(3),(5)-Hydroxyneoflavene and O-methyl derivatives&& | ||
| + | &&FLNE49NS&&Simple substitution&& | ||
| + | &&FLNF&&Coumarinic acid&& | ||
| + | &&FLNF19&&2,4,(3'),(5')-Hydroxycoumarinic acid&& | ||
| + | &&FLNF19NP&&Pyranoflavonoid&& | ||
| + | &&FLNFA9&&2,4,6,(3'),(5')-Hydroxycoumarinic acid&& | ||
| + | &&FLNFA9NP&&Pyranoflavonoid&& | ||
| + | ---> | ||
Revision as of 19:25, 24 May 2012
Neoflavonoid
| Flavonoid Top | Molecule Index | Author Index | Journals | Structure Search | Food | New Input |
Upper classes : FL Flavonoid
Overview
| 2nd Class | |||||
|---|---|---|---|---|---|
| FLNA | 4-Arylcoumarin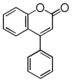
|
FLNB | 4-Arylchroman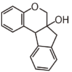
|
FLNC | Dalbergiquinol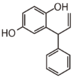
|
| FLND | Dalbergione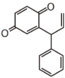
|
FLNE | Neoflavene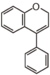
|
FLNF | Coumarinic acid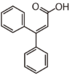
|
Database statistics
Major Plant Families
|
|
The number in each family is counted as the number of genera (not species) listed in our registered references. Each reference record is accessible by clicking the link in compound pages. The taxonomy follows the APG-II classification. For details (or if the figure is broken), visit this page. 各科のカウントは種名でなく文献に記載された属名の数です。文献は代謝物ページのリンクからたどれ、分類はAPG-IIです。左の図が表示されない場合はここをクリックしてください。 |