Category:FL6
| (25 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| − | + | {{Huge|Flavan, Leucoanthocyanidin}} | |
| + | {{Hierarchy|{{PAGENAME}}}} | ||
| + | |||
| + | =={{Bilingual|フラバン・ロイコアントシアニジンの概要|Overview}}== | ||
| + | {| class="wikitable" border="1" cellspacing="0" | ||
| + | |- | ||
| + | ! colspan="2"| Flavan | ||
| + | ! colspan="2"| Flavanol | ||
| + | |- | ||
| + | |[[:Category:FL6F|FL6F]] | ||
| + | |align="center"|[[:Category:FL6F|Flavan]]<br>[[Image:Fl6f.png|90px]]<br> hydroxy-, methoxyflavan, diffutidin etc. | ||
| + | |[[:Category:FL63|FL63]] | ||
| + | |align="center"|[[:Category:FL63|Flavan 3-ol]]<br>[[Image:Fl63.png|90px]]<br> (epi)catechin, (epi)fisetinidol, mesquitol, etc. | ||
| + | |- | ||
| + | ! colspan="4"| Leucoanthocyanidin | ||
| + | |- | ||
| + | |[[:Category:FL64|FL64]] | ||
| + | |align="center"|[[:Category:FL64|Flavan 4-ol]]<br>[[Image:Fl64.png|90px]]<br> apiforol, luteoforol etc. | ||
| + | |[[:Category:FL6D|FL6D]] | ||
| + | |align="center"|[[:Category:FL6D|Flavan 3,4-diol]]<br>[[Image:Fl6d.png|90px]]<br> leucoanthocyanidin etc. | ||
| + | |} | ||
| + | |||
| + | '''{{Bilingual|カテキン|Catechin}}''' | ||
| + | {{Twocolumn| | ||
| + | Catechins refer to a subgroup of [[:Category:FL63|Flavan 3-ol]] derivatives (FL63AC). In a stricter sense, it means [[FL63ACNS0001|(+)-Catechin]] and its stereoisomer | ||
| + | [[FL63ACNS0002|(-)-Epicatechin]]. | ||
| + | | | ||
| + | いわゆるカテキン類とは、[[:Category:FL63|Flavan 3-ol]]の下にあるグループを指します。狭義には[[FL63ACNS0001|(+)-Catechin]]とその立体異性体である | ||
| + | [[FL63ACNS0002|(-)-Epicatechin]]を指します。 | ||
| + | }} | ||
| + | |||
| + | '''{{Bilingual|ロイコアントシアニジン|Leucoanthocyanidin}}''' | ||
| + | {{Twocolumn| | ||
| + | [[:Category:FL63|Flavan 3-ol]] is often called flavanol. | ||
| + | [[:Category:FL6D|Flavan 3,4-diol]] and [[:Category:FL64|Flavan 4-ol]] are called leucoanthocyanidin meaning a precursor (white state) of (colored) anthocyans. The term was first used by Rosenheim in 1920. | ||
| + | ''Leuco'' comes from the Greek word λευκός (leukos) meaning white. | ||
| + | <br/> | ||
| + | In scientific terms, leucoanthocyanidins refer to monomers, which are converted to anthocyanidins by 2-oxoglutarate iron-dependent oxygenase (i.e., non-haem oxygenase) called ''anthocyanidin synthase''. | ||
| + | | | ||
| + | [[:Category:FL63|Flavan 3-ol]] はしばしばフラバノールと呼ばれます。 | ||
| + | [[:Category:FL6D|Flavan 3,4-diol]] と [[:Category:FL64|Flavan 4-ol]] はロイコアントシアニジンと呼ばれ、 | ||
| + | 植物色素アントシアンの前駆体です。この言葉をはじめて用いたのはRosenheim (1920)で、"ロイコ"とはギリシャ語λευκόςで「白」を意味します<ref>Rosenheim O (1920) ''Biochem. J.'', 14, 178-188</ref>。 | ||
| + | <br/> | ||
| + | より詳しくいうと、ロイコアントシアニジンは単量体を意味し、アントシアニジン合成酵素と呼ばれる2-オキソグルタル酸、鉄依存型オキシゲナーゼ(ヘム非依存型)によりアントシアニジンへ変換されます。 | ||
| + | }} | ||
| + | |||
| + | '''{{Bilingual|プロアントシアニジン・プロシアニジン|Proanthocyanidin, procyanidin}}''' | ||
| + | {{Twocolumn| | ||
| + | Proanthocyanidins are polymers of [[:Category:FL63|flavan 3-ol]], up to 50 units. They are cleaved to anthocyanidins under strongly acidic conditions. | ||
| + | Proanthocyanidins that consist exclusively of (epi)catechins are called also procyanidins. | ||
| + | | | ||
| + | プロアントシアニジンという言葉がありますが、こちらは[[:Category:FL63|flavan 3-ol]] のポリマーを意味し(最大で50ユニット程度)、強酸下でアントシアニジンに分解されます。 | ||
| + | (エピ)カテキンのみから構成されるプロアントシアニジンは、プロシアニジンとも呼ばれます。 | ||
| + | }} | ||
| + | <references/> | ||
| + | |||
| + | ==Database statistics データベース統計==<!--- see FL1---> | ||
| + | {{#def:FL6|{{#SearchTitle:FL6|}}}} | ||
| + | {{#def:rFL6|{{#SearchLine:&&FL6|Reference}}}} | ||
| + | {{#graph:pie|size=350x200;title=Registered Molecules | ||
| + | ({{#count:{{#var:FL6}}}} total);legend=0.01x0.2;label=; | ||
| + | FL6F={{#count:{{#choose:{{#var:FL6}}|FL6F}}}}; | ||
| + | FL63={{#count:{{#choose:{{#var:FL6}}|FL63}}}}; | ||
| + | FL64={{#count:{{#choose:{{#var:FL6}}|FL64}}}}; | ||
| + | FL1D={{#count:{{#choose:{{#var:FL6}}|FL6D}}}}; | ||
| + | }} | ||
| + | {{#graph:pie|size=350x200;title=Metabolite-Species Data ({{#countLine:&&FL6|Reference}} total);legend=0.01x0.2;label=; | ||
| + | FL6F={{#count:{{#choose:{{#var:rFL6}}|&&FL6F}}}}; | ||
| + | FL63={{#count:{{#choose:{{#var:rFL6}}|&&FL63}}}}; | ||
| + | FL64={{#count:{{#choose:{{#var:rFL6}}|&&FL64}}}}; | ||
| + | FL6D={{#count:{{#choose:{{#var:rFL6}}|&&FL6D}}}}; | ||
| + | }} | ||
| + | |||
| + | {{FL_pie_chart|FL6}} | ||
[[Category:FL]] | [[Category:FL]] | ||
| + | |||
| + | <!--- Class Information (Do not delete. It is used by Template:Hierarchy.) | ||
| + | &&FL6&&Flavan&& | ||
| + | &&FL63&&Flavan 3-ol&& | ||
| + | &&FL631A&&Guibourtinidol and O-methyl derivatives&& | ||
| + | &&FL631ANI&&Non-cyclic prenyl substituted&& | ||
| + | &&FL631ANM&&C-Methyl or C2/C3 substituted&& | ||
| + | &&FL631ANS&&Simple substitution&& | ||
| + | &&FL631C&&Fisetinidol, Epifisetinidol and O-methyl derivatives&& | ||
| + | &&FL631CGS&&O-Glycoside&& | ||
| + | &&FL631CNC&&Flavonoid substituted by complex substituent&& | ||
| + | &&FL631CNS&&Simple substitution&& | ||
| + | &&FL631G&&Robinetinidol, Entrobinetinidol and O-methyl derivatives&& | ||
| + | &&FL631GNC&&Flavonoid substituted by complex substituent&& | ||
| + | &&FL631GNS&&Simple substitution&& | ||
| + | &&FL633C&&Mesquitol, Epimesquitol and O-methylderivatives&& | ||
| + | &&FL633CNS&&Simple substitution&& | ||
| + | &&FL63A8&&3,5,7,2',(3'),(5'),(6')-Hydroxyflavan&& | ||
| + | &&FL63A8NS&&Simple substitution&& | ||
| + | &&FL63A9&&3,5,7,(3'),(5')-Hydroxyflavan&& | ||
| + | &&FL63A9NS&&Simple substitution&& | ||
| + | &&FL63AA&&Afzelechin and Epiafzelechin&& | ||
| + | &&FL63AAGS&&O-Glycoside&& | ||
| + | &&FL63AANM&&C-Methyl or C2/C3 substituted&& | ||
| + | &&FL63AANS&&Simple substitution&& | ||
| + | &&FL63AC&&Catechin and Epicatechin&& | ||
| + | &&FL63ACCS&&C-Glycoside&& | ||
| + | &&FL63ACGC&&Flavonoid O-glycoside substituted by complex substituent&& | ||
| + | &&FL63ACGCN&&Nitrogen included&& | ||
| + | &&FL63ACGN&&Flavonophenylpropanoid O-glycoside&& | ||
| + | &&FL63ACGS&&O-Glycoside&& | ||
| + | &&FL63ACNC&&Flavonoid substituted by complex substituent&& | ||
| + | &&FL63ACNI&&Non-cyclic prenyl substituted&& | ||
| + | &&FL63ACNM&&C-Methyl or C2/C3 substituted&& | ||
| + | &&FL63ACNN&&Flavonophenylpropanoid&& | ||
| + | &&FL63ACNP&&Pyranoflavonoid&& | ||
| + | &&FL63ACNS&&Simple substitution&& | ||
| + | &&FL63AD&&Symplocosidin&& | ||
| + | &&FL63ADGS&&O-Glycoside&& | ||
| + | &&FL63ADNS&&Simple substitution&& | ||
| + | &&FL63AE&&Catechin 4'-methyl ether and Epicatechin 4'-methyl ether&& | ||
| + | &&FL63AEGS&&O-Glycoside&& | ||
| + | &&FL63AENS&&Simple substitution&& | ||
| + | &&FL63AF&&Catechin 3',4'-dimethyl ether and Epicatechin 3',4'-dimethyl ether&& | ||
| + | &&FL63AFNS&&Simple substitution&& | ||
| + | &&FL63AG&&Gallocatechin and Epigallocatechin&& | ||
| + | &&FL63AGGC&&Flavonoid O-glycoside substituted by complex substituent&& | ||
| + | &&FL63AGNN&&Flavonophenylpropanoid&& | ||
| + | &&FL63AGNS&&Simple substitution&& | ||
| + | &&FL63AJ&&Gallocatechin 4'-methyl ether and Epigallocatechin 4'-methyl ehter&& | ||
| + | &&FL63AJNS&&Simple substitution&& | ||
| + | &&FL63BA&&Afzelechin 5-methyl ether&& | ||
| + | &&FL63BANI&&Non-cyclic prenyl substituted&& | ||
| + | &&FL63BANP&&Pyranoflavonoid&& | ||
| + | &&FL63BB&&Afzelechin 5,4'-dimethyl ether&& | ||
| + | &&FL63BBNS&&Simple substitution&& | ||
| + | &&FL63BD&&Epicatechin 5,3'-dimethyl ether&& | ||
| + | &&FL63BDNS&&Simple substitution&& | ||
| + | &&FL63BI&&Epigallocatechin 5,3',5'-trimethyl ether&& | ||
| + | &&FL63BINS&&Simple substitution&& | ||
| + | &&FL63CC&&Catechin 7-methyl ether&& | ||
| + | &&FL63CCNS&&Simple substitution&& | ||
| + | &&FL63CD&&Catechin 3'-methyl ether&& | ||
| + | &&FL63CDNS&&Simple substitution&& | ||
| + | &&FL63CE&&Catechin 7,4'-dimethyl ether&& | ||
| + | &&FL63CENS&&Simple substitution&& | ||
| + | &&FL63CI&&Gallocatechin 7,3',5'-trimethyl ether&& | ||
| + | &&FL63CIGS&&O-Glycoside&& | ||
| + | &&FL63DC&&Epicatechin 5,7-dimethyl ether&& | ||
| + | &&FL63DCNS&&Simple substitution&& | ||
| + | &&FL63DD&&Catechin 5,7,3'-trimethyl ether and Epicatechin 5,7,3'-trimethyl ether&& | ||
| + | &&FL63DDNS&&Simple substitution&& | ||
| + | &&FL63DE&&Catechin 5,7,4'-trimethyl ether&& | ||
| + | &&FL63DENS&&Simple substitution&& | ||
| + | &&FL63EC&&3,5,6,7,3',4'-Hexahydroxyflavan&& | ||
| + | &&FL63ECGS&&O-Glycoside&& | ||
| + | &&FL63FC&&3,5,7,8,3',4'-Hexahydroxyflavan&& | ||
| + | &&FL63FCGS&&O-Glycoside&& | ||
| + | &&FL63GC&&Elephantorrhizol&& | ||
| + | &&FL63GCNS&&Simple substitution&& | ||
| + | &&FL63PT&&Peltogynoid flavan&& | ||
| + | &&FL63PTNM&&C-Methyl or C2/C3 substituted&& | ||
| + | &&FL63PTNS&&Simple substitution&& | ||
| + | &&FL63RN&&4-O-2' cycled flavan 3-ol && | ||
| + | &&FL63RNNS&&Simple substitution&& | ||
| + | &&FL64&&Flavan 4-ol&& | ||
| + | &&FL64A9&&4,5,7,(3'),(5')-Hydroxyflavan&& | ||
| + | &&FL64A9GM&&C-Methyl or C2/C3 substituted flavononoid O-glycoside&& | ||
| + | &&FL64A9NF&&Furanoflavonoid&& | ||
| + | &&FL64A9NI&&Non-cyclic prenyl substituted&& | ||
| + | &&FL64A9NP&&Pyranoflavonoid&& | ||
| + | &&FL64AA&&Apiforol&& | ||
| + | &&FL64AAGM&&C-Methyl or C2/C3 substituted flavononoid O-glycoside&& | ||
| + | &&FL64AANS&&Simple substitution&& | ||
| + | &&FL64AB&&Apiforol 4-methyl ether&& | ||
| + | &&FL64ABGM&&C-Methyl or C2/C3 substituted flavononoid O-glycoside&& | ||
| + | &&FL64AC&&Luteoforol&& | ||
| + | &&FL64ACNS&&Simple substitution&& | ||
| + | &&FL64CA&&Apiforol 7-methyl ether&& | ||
| + | &&FL64CANI&&Non-cyclic prenyl substituted&& | ||
| + | &&FL64DB&&Apiforol 5,7,4'-trimethyl ehter&& | ||
| + | &&FL64DBGM&&C-Methyl or C2/C3 substituted flavononoid O-glycoside&& | ||
| + | &&FL64DBNS&&Simple substitution&& | ||
| + | &&FL64RN&&4-O-2' Cycled flavan 4-ol&& | ||
| + | &&FL64RNNP&&Pyranoflavonoid&& | ||
| + | &&FL6D&&Flavan 3,4-diol&& | ||
| + | &&FL6D1A&&Guibourtinidol 4-ol, Epiguibourtinidol 4-ol and O-methyl derivatives&& | ||
| + | &&FL6D1ANS&&Simple substitution&& | ||
| + | &&FL6D1C&&Fisetinidol 4-ol, Epifisetinidol 4-ol and O-methyl derivatives&& | ||
| + | &&FL6D1CNS&&Simple substitution&& | ||
| + | &&FL6D1G&&Robinetinidol 4-ol&& | ||
| + | &&FL6D1GNS&&Simple substitution&& | ||
| + | &&FL6D3A&&Oritin 4-ol, Epioritin 4-ol and O-methyl derivatives&& | ||
| + | &&FL6D3ANS&&Simple substitution&& | ||
| + | &&FL6D3C&&Mesquitol 4-ol, Epimesquitol 4-ol and O-methyl derivatives&& | ||
| + | &&FL6D3CNS&&Simple substitution&& | ||
| + | &&FL6DA9&&3,4,5,7,(3'),(5')-Hydroxyflavan and O-methylderivatives&& | ||
| + | &&FL6DA9NP&&Pyranoflavonoid&& | ||
| + | &&FL6DAA&&Leucopelargonidin&& | ||
| + | &&FL6DAAGS&&O-Glycoside&& | ||
| + | &&FL6DAC&&Leucocyanidin&& | ||
| + | &&FL6DACGS&&O-Glycoside&& | ||
| + | &&FL6DACNS&&Simple substitution&& | ||
| + | &&FL6DAD&&Leucocyanidin 3'-methyl ether&& | ||
| + | &&FL6DADGS&&O-Glycoside&& | ||
| + | &&FL6DAG&&Leucodelphinidin&& | ||
| + | &&FL6DAGGS&&O-Glycoside&& | ||
| + | &&FL6DAGNS&&Simple substitution&& | ||
| + | &&FL6DBA&&Leucopelargonidin 5-methyl ether&& | ||
| + | &&FL6DBAGI&&Non-cyclic prenyl substituted flavonoid O-glycoside&& | ||
| + | &&FL6DBANI&&Non-cyclic prenyl substituted&& | ||
| + | &&FL6DBANP&&Pyranoflavonoid&& | ||
| + | &&FL6DBB&&Leucopelargonidin 5,4'-dimethyl ether&& | ||
| + | &&FL6DBBNM&&C-Methyl or C2/C3 substituted&& | ||
| + | &&FL6DDA&&Leocopelargonidin O-methyl derivatives (4'-Hydroxy, without FL6DBA)&& | ||
| + | &&FL6DDAGI&&Non-cyclic prenyl substituted flavonoid O-glycoside&& | ||
| + | &&FL6DDANI&&Non-cyclic prenyl substituted&& | ||
| + | &&FL6DDANP&&Pyranoflavonoid&& | ||
| + | &&FL6DE9&&3,4,5,6,7,(3'),(5')-Hydroxyflavan and O-methyl derivatives&& | ||
| + | &&FL6DE9NF&&Furanoflavonoid&& | ||
| + | &&FL6DF9&&3,4,5,7,8,(3'),(5')-Hydroxyflavan and O-methyl derivatives&& | ||
| + | &&FL6DF9NF&&Furanoflavonoid&& | ||
| + | &&FL6DPT&&Peltogynoid flavan 4-ol&& | ||
| + | &&FL6DPTNS&&Simple substitution&& | ||
| + | &&FL6F&&Flavan&& | ||
| + | &&FL6F19&&7,(3'),(5')-Hydroxyflavan&& | ||
| + | &&FL6F19NS&&Simple substitution&& | ||
| + | &&FL6F1A&&Cassiaflavan and O-methyl derivatives&& | ||
| + | &&FL6F1ANC&&Flavonoid substituted by complex substituent&& | ||
| + | &&FL6F1ANM&&C-Methyl or C2/C3 substituted&& | ||
| + | &&FL6F1ANS&&Simple substitution&& | ||
| + | &&FL6F1C&&7,3',4'-Trihydroxyflavan and O-methyl derivatives&& | ||
| + | &&FL6F1CGS&&O-Glycoside&& | ||
| + | &&FL6F1CNI&&Non-cyclic prenyl substituted&& | ||
| + | &&FL6F1CNM&&C-Methyl or C2/C3 substituted&& | ||
| + | &&FL6F1CNP&&Pyranoflavonoid&& | ||
| + | &&FL6F1CNS&&Simple substitution&& | ||
| + | &&FL6F1G&&7,3',4',5'-Tetrahydroxyflavan and O-methylderivatives&& | ||
| + | &&FL6F1GGS&&O-Glycoside&& | ||
| + | &&FL6F1L&&7,2',4'-Hydroxyflavan&& | ||
| + | &&FL6F1LNM&&C-Methyl or C2/C3 substituted&& | ||
| + | &&FL6F1LNS&&Simple substitution&& | ||
| + | &&FL6FA9&&5,7,(3'),(5')-Hydroxyflavan&& | ||
| + | &&FL6FA9GS&&O-Glycoside&& | ||
| + | &&FL6FA9NC&&Flavonoid substituted by complex substituent&& | ||
| + | &&FL6FA9NI&&Non-cyclic prenyl substituted&& | ||
| + | &&FL6FA9NM&&C-Methyl or C2/C3 substituted&& | ||
| + | &&FL6FA9NS&&Simple substitution&& | ||
| + | &&FL6FAA&&Apigeniflavan&& | ||
| + | &&FL6FAAGS&&O-Glycoside&& | ||
| + | &&FL6FAANS&&Simple substitution&& | ||
| + | &&FL6FAB&&5,7-Dihydroxy-4'-methoxyflavan&& | ||
| + | &&FL6FABNS&&Simple substitution&& | ||
| + | &&FL6FAC&&5,7,3',4'-Tetrahydroxyflavan&& | ||
| + | &&FL6FACGS&&O-Glycoside&& | ||
| + | &&FL6FAF&&Diffutidin&& | ||
| + | &&FL6FAFGS&&O-Glycoside&& | ||
| + | &&FL6FAFNS&&Simple substitution&& | ||
| + | &&FL6FAL&&5,7,2',4'-Hydroxyflavan&& | ||
| + | &&FL6FALNM&&C-Methyl or C2/C3 substituted&& | ||
| + | &&FL6FBB&&7-Hydroxy-5,4'-dimethoxyflavan&& | ||
| + | &&FL6FBBNS&&Simple substitution&& | ||
| + | &&FL6FCA&&5,4'-Dihydroxy-7-methoxyflavan&& | ||
| + | &&FL6FCANM&&C-Methyl or C2/C3 substituted&& | ||
| + | &&FL6FCB&&5-Hydroxy-7,4'-dimethoxyflavan&& | ||
| + | &&FL6FCBGS&&O-Glycoside&& | ||
| + | &&FL6FCF&&5-Hydroxy-7,3',4'-trimethoxyflavan&& | ||
| + | &&FL6FCFGS&&O-Glycoside&& | ||
| + | &&FL6FDA&&4'-Hydroxy-5,7-dimethoxyflavan&& | ||
| + | &&FL6FDANC&&Flavonoid substituted by complex substituent&& | ||
| + | &&FL6FDANM&&C-Methyl or C2/C3 substituted&& | ||
| + | &&FL6FDANS&&Simple substitution&& | ||
| + | &&FL6FDB&&5,7,4'-Trimethoxyflavan&& | ||
| + | &&FL6FDBNS&&Simple substitution&& | ||
| + | &&FL6FDC&&3',4'-Dihydroxy-5,7-dimethoxyflavan&& | ||
| + | &&FL6FDCNS&&Simple substitution&& | ||
| + | &&FL6FDD&&4'-Hydroxy-5,7,3'-trimethoxyflavan&& | ||
| + | &&FL6FDDNS&&Simple substitution&& | ||
| + | &&FL6FHY&&2-Hydroxyflavan&& | ||
| + | &&FL6FHYNI&&Non-cyclic prenyl substituted&& | ||
| + | &&FL6FUN&&Unusual core flavan&& | ||
| + | &&FL6FUNNI&&Non-cyclic prenyl substituted&& | ||
| + | &&FL6FWX&&Flaven&& | ||
| + | &&FL6FWXNF&&Furanoflavonoid&& | ||
| + | &&FL6FWXNI&&Non-cyclic prenyl substituted&& | ||
| + | ---> | ||
Latest revision as of 10:14, 19 August 2010
Flavan, Leucoanthocyanidin
| Flavonoid Top | Molecule Index | Author Index | Journals | Structure Search | Food | New Input |
Upper classes : FL Flavonoid
[edit] Overview
| Flavan | Flavanol | ||
|---|---|---|---|
| FL6F | Flavan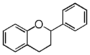 hydroxy-, methoxyflavan, diffutidin etc. |
FL63 | Flavan 3-ol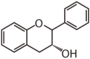 (epi)catechin, (epi)fisetinidol, mesquitol, etc. |
| Leucoanthocyanidin | |||
| FL64 | Flavan 4-ol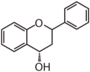 apiforol, luteoforol etc. |
FL6D | Flavan 3,4-diol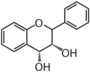 leucoanthocyanidin etc. |
Catechin
Catechins refer to a subgroup of Flavan 3-ol derivatives (FL63AC). In a stricter sense, it means (+)-Catechin and its stereoisomer (-)-Epicatechin.
Leucoanthocyanidin
Flavan 3-ol is often called flavanol.
Flavan 3,4-diol and Flavan 4-ol are called leucoanthocyanidin meaning a precursor (white state) of (colored) anthocyans. The term was first used by Rosenheim in 1920.
Leuco comes from the Greek word λευκός (leukos) meaning white.
In scientific terms, leucoanthocyanidins refer to monomers, which are converted to anthocyanidins by 2-oxoglutarate iron-dependent oxygenase (i.e., non-haem oxygenase) called anthocyanidin synthase.
Proanthocyanidin, procyanidin
Proanthocyanidins are polymers of flavan 3-ol, up to 50 units. They are cleaved to anthocyanidins under strongly acidic conditions. Proanthocyanidins that consist exclusively of (epi)catechins are called also procyanidins.
[edit] Database statistics データベース統計
[edit] Major Plant Families
|
|
The number in each family is counted as the number of genera (not species) listed in our registered references. Each reference record is accessible by clicking the link in compound pages. The taxonomy follows the APG-II classification. For details (or if the figure is broken), visit this page. 各科のカウントは種名でなく文献に記載された属名の数です。文献は代謝物ページのリンクからたどれ、分類はAPG-IIです。左の図が表示されない場合はここをクリックしてください。 |