Category:FL3F
m (→{{Bilingual|フラボンの概要|Overview}}) |
|||
| (6 intermediate revisions by one user not shown) | |||
| Line 2: | Line 2: | ||
{{Hierarchy|{{PAGENAME}}}} | {{Hierarchy|{{PAGENAME}}}} | ||
| + | |||
| + | =={{Bilingual|フラボンの概要|Overview}}== | ||
| + | |||
{{Twocolumn| | {{Twocolumn| | ||
The basic structure of flavone is 2-phenylbenzopyrone, the same as [[:Category:FL5|flavonol]]. | The basic structure of flavone is 2-phenylbenzopyrone, the same as [[:Category:FL5|flavonol]]. | ||
The word 'flavone' first appeard in 1895<ref>S. von Kostanecki and J. Jambor, Ber. Deut. Chem. Ges. 28, 2302, 1895</ref>, indicating its yellow color. | The word 'flavone' first appeard in 1895<ref>S. von Kostanecki and J. Jambor, Ber. Deut. Chem. Ges. 28, 2302, 1895</ref>, indicating its yellow color. | ||
| − | [[ | + | [[FL3FACNS0001|luteolin]], [[FL5FACNS0001|quercetin]], [[FL5FAANS0001|kaempferol]] were widespread natural dyes before synthetic dyes were invented in 1856 <ref>J. Piccard, Ber. Deut. Chem. Ges. 6, 884 (1873); 7, 888 (1874); 10, 176 (1877)</ref>. As a dye, flavone requires a mordant to chelate positions 4 and 5 with metallic salts. Ferric chloride gives green with 5-hydroxyflavones and brown with 3-hydroxyflavones (flavonol). |
| + | |||
| + | [[FL3FA9NS0002|Chrysin]] was the first flavone to be isolated in a pure form. Many members in [[:Category:Asteraceae|Asteraceae]] contain unique flavones, especially the genus [[Species:Cirsium|Cirsium]] and [[Species:Onopordum|Onopordum]] in the tribe Cardueae (or equivalently, Cynareae), commonly known as thistles. | ||
| | | | ||
フラボンの骨格は2-phenylbenzopyroneといい、[[:Category:FL5|フラボノール]]と共通です(右下図)。 | フラボンの骨格は2-phenylbenzopyroneといい、[[:Category:FL5|フラボノール]]と共通です(右下図)。 | ||
| − | + | フラボンという言葉は1895年に、黄色の染料として使われました。[[FL3FACNS0001|ルテオリン]] (flavone)、[[FL5FACNS0001|クエルセチン]]と[[FL5FAANS0001|ケンフェロール]](flavonol)はいずれも1856年に合成色素が発明されるまでは広く使われた黄色の染料でした。色素として使うには金属塩の媒染剤が必要です。5-ヒドロキシの場合は塩化鉄で緑、3-ヒドロキシの場合 (flavonol) は茶色になります。 | |
| − | + | ||
| + | [[FL3FA9NS0002|クリシン]]は一番最初に単離されたフラボンです。[[:Category:Asteraceae|キク科]]には特徴的なflavoneが多く、一般にはアザミとして知られるCardueae(別名Cynareae)属の[[Species:Cirsium|Cirsium]]や[[Species:Onopordum|Onopordum]]では多くのflavoneが見つかっています。 | ||
}} | }} | ||
| + | |||
| + | '''{{Bilingual|ポリメトキシフラボン|Polymethoxylated flavones}}''' | ||
| + | {{Twocolumn| | ||
| + | Most flavones are not widely distributed and often 7-O-glycosylated. | ||
| + | Major dietary sources are celery, parsley, and some herbs. | ||
| + | In citrus species, polymethoxylated flavones such as [[FL3FGANS0002|tangeretin]] and [[FL3FGCNS0001|nobiletin]] are found. | ||
| + | | | ||
| + | ほとんどのフラボンは限定された植物種に含まれ、その多くが7位に糖がつきます。 | ||
| + | フラボンを含む主な食品はセロリ、パセリなどのハーブです。 | ||
| + | 柑橘類からは、[[FL3FGANS0002|タンジェレチン]]や[[FL3FGCNS0001|ノビレチン]]などのポリメトキシフラボンが見つかっています。 | ||
| + | }} | ||
| + | |||
| + | <center> | ||
| + | {| | ||
| + | | [[FL3FAANS0001|apigenin]] <br/>[[Image:FL3FAANS0001.png|100px]] | ||
| + | | [[FL3FACNS0001|luteolin]] <br/>[[Image:FL3FACNS0001.png|100px]] | ||
| + | | [[FL3FGANS0002|tangeretin]] <br/>[[Image:FL3FGANS0002.png|100px]] | ||
| + | | [[FL3FGCNS0001|nobiletin]] <br/>[[Image:FL3FGCNS0001.png|100px]] | ||
| + | |} | ||
| + | </center> | ||
==Structure== | ==Structure== | ||
Latest revision as of 11:58, 15 July 2011
FL3F: Flavone
| Flavonoid Top | Molecule Index | Author Index | Journals | Structure Search | Food | New Input |
Upper classes : FL Flavonoid : FL3 Flavone
Contents |
[edit] Overview
The basic structure of flavone is 2-phenylbenzopyrone, the same as flavonol. The word 'flavone' first appeard in 1895[1], indicating its yellow color. luteolin, quercetin, kaempferol were widespread natural dyes before synthetic dyes were invented in 1856 [2]. As a dye, flavone requires a mordant to chelate positions 4 and 5 with metallic salts. Ferric chloride gives green with 5-hydroxyflavones and brown with 3-hydroxyflavones (flavonol). Chrysin was the first flavone to be isolated in a pure form. Many members in Asteraceae contain unique flavones, especially the genus Cirsium and Onopordum in the tribe Cardueae (or equivalently, Cynareae), commonly known as thistles.
Polymethoxylated flavones
Most flavones are not widely distributed and often 7-O-glycosylated. Major dietary sources are celery, parsley, and some herbs. In citrus species, polymethoxylated flavones such as tangeretin and nobiletin are found.
apigenin 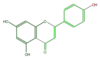
|
luteolin 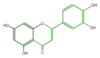
|
tangeretin 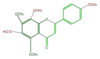
|
nobiletin 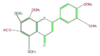
|
[edit] Structure
Flavones are commonly present in vacuoles as O- and/or C-glycosides. Most glycosides are 7-, 3'-, and 4'-glycosides. 5-glycoside is rare because the 5-hydroxyl group forms a hydrogen bond with the adjacent 4-carbonyl group. Flavone glycosides have been frequently used as chemotaxonomic markers[3].
Isovitexin (C-glycosylflavone) acts as co-pigments of anthocyanins in Iris ensata Thunb. (Japanese iris flower)[4]
- ↑ S. von Kostanecki and J. Jambor, Ber. Deut. Chem. Ges. 28, 2302, 1895
- ↑ J. Piccard, Ber. Deut. Chem. Ges. 6, 884 (1873); 7, 888 (1874); 10, 176 (1877)
- ↑ Iwashina T & Hatta H: Flavonoid glycosides from leaves of Aucuba japonica and Helwingia japonica: Phytochemical relationship with the genus Cornus J. Jap. Bot. 72:337-346, 1997
Reference:Iwashina_T:Ootani_S:,Phytochemistry,1990,29,3639
Reference:Iwashina T:Kamenosono K:Ueno T:,Phytochemistry,1999,51,1109 - ↑ Iwashina T, Kamenosono K, and Yabuya T: Isolation and identification of flavonoid and related compounds as co-pigments from the flowers of Iris ensata. J. Jap. Bot. 71:281-287
[edit] Major Plant Families
|
|
The number in each family is counted as the number of genera (not species) listed in our registered references. Each reference record is accessible by clicking the link in compound pages. The taxonomy follows the APG-II classification. For details (or if the figure is broken), visit this page. 各科のカウントは種名でなく文献に記載された属名の数です。文献は代謝物ページのリンクからたどれ、分類はAPG-IIです。左の図が表示されない場合はここをクリックしてください。 |
[edit] Patterns of Hydroxylation
| Category Names in the 3rd Class | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
The 5th and 6th digits of our flavonoid ID indicates the hydroxylation patterns of A-ring and B-ring (See the upper-right figure. The leftmost ring is A, rightmost is B), respectively. The following chart is spanned by these 2 digits. R indicates H or CH3, and R' indicates H or R. Numbers are IUPAC positions. The value in each cell (such as GS: glycosilation only) corresponds to the 7th and 8th digits, which is explained at the bottom of this page.
|
Position of -OH (-OCH3) groups |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
| A (443 pages) | B (53 pages) | C (395 pages) | D (72 pages) | E (33 pages) | F (11 pages) | G (77 pages) | H (4 pages) | I (23 pages) | J (2 pages) | K (17 pages) | L (104 pages) | 8 (83 pages) | 9 (128 pages) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A (776 pages) | |||||||||||||||
| B (15 pages) | |||||||||||||||
| C (101 pages) | |||||||||||||||
| D (8 pages) | |||||||||||||||
| E (213 pages) | |||||||||||||||
| F (135 pages) | |||||||||||||||
| G (88 pages) | |||||||||||||||
| 1 (50 pages) | |||||||||||||||
| 2 (16 pages) | |||||||||||||||
| 3 (6 pages) | |||||||||||||||
| 4 (1 pages) | |||||||||||||||
| 9 (36 pages) | |||||||||||||||
Abbreviations used in the above chart
- First Characters
N not glycosylated; G O-glycoside; C C-glycoside; D both glycosides;
- Second Characters
S not modified; M alkylated; I prenylated; R cyclic-prenylated; F furanoFL; P pyranoFL; D furano and pyranoFL; N phenylpropanoid; C others;
- Special Second Character only for Anthycyanin (FL7)
A Galactosylated; L Glucosylated; O modified with other sugars;
[edit] Other Unusual Patterns
These types are not classified in the above chart.
| Quinone | QU (6 pages) | alpha-Hydroxy | HX (0 pages) | beta-Hydroxy | HY (0 pages) | Peltogynoid | PT (0 pages) |
| Retrocalchone | RT (0 pages) | Dehydro-backbone | WX (0 pages) | Additional rings | RN (28 pages) | Others | UN (0 pages) |
| Pyranoanthocyanin (FL7 only) | RX (0 pages) | ||||||
[edit] Patterns of Glycosylation
The 7th and 8th digits of the flavonoid ID indicates the glycosylation, and other modification patterns, respectively. The following chart is spanned by these 2 digits. The value in each cell (such as AA for the standard form) corresponds to the 5th and 6th digits.
| 7th digit → 8th digit ↓ |
No glycosylation N (551 pages) | O-glycoside G (549 pages) | C-glycoside C (288 pages) | O- & C-glycoside D (91 pages) | |
|---|---|---|---|---|---|
| no modification | S (1255 pages) | ||||
| alkylated | M (27 pages) | ||||
| prenylated | I (0 pages) | ||||
| cyclic prenylated | R (0 pages) | ||||
| furano FL | F (30 pages) | ||||
| pyrano FL | P (64 pages) | ||||
| furano & pyrano FL | D (9 pages) | ||||
| prenylpropanoid | N (5 pages) | ||||
| others | C (9 pages) | ||||
| 3-Gal related | A | N.A. | N.A. | N.A. | |
| 3-Glc related | L | N.A. | N.A. | N.A. | |
| other sugar at 3 | O | N.A. | N.A. | N.A. | |
This category currently contains no pages or media.