Index:PK
From Metabolomics.JP
| name | PKS | gene | organism | Ref | Note | Size | C2 | ||
|---|---|---|---|---|---|---|---|---|---|
| 1,3,8-Trihydroxyaceto-Naphthalene | Aspergillus parvulus | Herbert1989 | Compound name have not been confirmed. Naphthalene is the base structure of statins. | 12 | 6 | 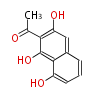
| |||
| 1,3-Dihydroxy-N-Methylacridone | Acridone synthase | Dewick2009 | acridine alkaloid, starter is Anthranilic acid | 3 | 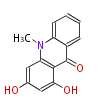
| ||||
| 3,8-Dihydroxy-1-Methylanthraquinone-2-Carboxylic Acid | Staunton&Weissman2001 | 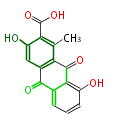
| |||||||
| 4-Hydroxy-2-Quinolone | Dewick2009 | quinoline alkaloid, starter is Anthranilic acid | 1 | 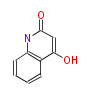
| |||||
| 5,7-Dihydroxy-2-Methylchromone | Pentaketide chromone synthase | Ammi visnaga | Dewick2009 | furochromones,aromatic pentaketide.The starter unitis derived by decarboxylation.precursor of the well known antiasthmatic furochromones, kehellin and visnagin.PCS,Pentaketide chromone synthase ,plant-speci?c type III PKS | Escherichia coli | 10 | 5 | 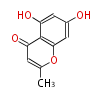
| |
| 5-Methylorsellinic Acid | Aspergillus flaviceps | Dewick2009 | C-methylated analogue of orsellinic acid,the extra methyl is derived from SAM. | 8 | 4 | 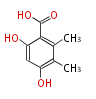
| |||
| 6-Deoxy-Erythronolide B | DEBS | Dewick2009 | 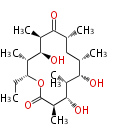
| ||||||
| 6-Methoxymellein | Dewick2009 | 5 | 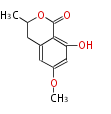
| ||||||
| 6-Methyl Salicylic Acid | 6-MSAS/P. patulum(ATX/Aspergillus terreus) | atX | Penicillium patulum, Aspergillus terreus | S. Gaisser, A. Trefzer, S. Stckert, A. Kirshning and A. Bechthold, J. Bacteriol., 1997, 179, 6271?6278.;Staunton&Weissman2001 | lack 2 OH group | Saccharomyces cerevisiae, E. coli | 8 | 4 | 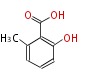
|
| Actinorhodin | act | Streptomyces coelicolor | Herbert1989 | Streptomyces parvulus | 16 | 8 | 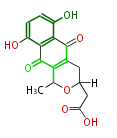
| ||
| Adhyperforin | Hypericum perforatum | Dewick2009 | 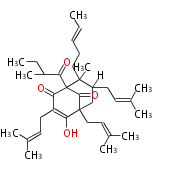
| ||||||
| Aflatoxin B1 | PKSL1/Aspergillus parasiticus=PKSA | pksL1/Aspergillus parasiticus (Fas1,Fas2,PksA,Nor-1,AvnA, AdhA, AvfA, EstA,VBS, VerB,AflN,AflM,OmtB,OmtA,OrdA) | Aspergillus flavus, Aspergillus parasiticus | Staunton&Weissman2001;Yabe&Nakajima2004;Dewick2009 | Norsolorinic acid and averufin are its precursors Hexanoate is the starter unit. Intermediate of Aflatoxin B and sterigmatocystin.The Aspergillus parasiticus polyketide synthase genepksA, a homolog of Aspergillus nidulans wA, is required for aflatoxin B1 biosynthesis | 14 | 10 | 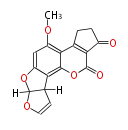
| |
| Aflatoxin B2 | Aspergillus flavus, Aspergillus parasiticus | Dewick2009 | Hexanoate is the starter unit | 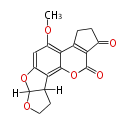
| |||||
| Aflatoxin G1 | (Fas1,Fas2,PksA,Nor-1,AvnA, AdhA, AvfA, EstA,VBS, VerB,AflN,AflM,OmtB,OmtA,OrdA) | Aspergillus flavus, Aspergillus parasiticus | Staunton&Weissman2001;Yabe&Nakajima2004;Dewick2009 | Norsolorinic acid and averufin are its precursors. Hexanoate is the starter unit. Intermediate of Aflatoxin B and sterigmatocystin. | 11 | 10 | 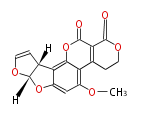
| ||
| Aflatoxin G2 | Aspergillus flavus, Aspergillus parasiticus | Dewick2009 | Hexanoate is the starter unit | 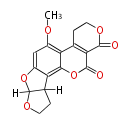
| |||||
| Aflatoxin M1 | Aspergillus flavus, Aspergillus parasiticus | Dewick2009 | Hexanoate is the starter unit | 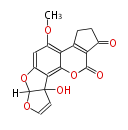
| |||||
| Aloe-Emodin | Aloe ferox | Dewick2009; | 15 | 8 | 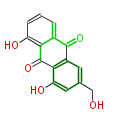
| ||||
| Aloesaponarin II | Staunton&Weissman2001;Yabe&Nakajima2004;Dewick2009 | 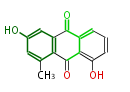
| |||||||
| Alternapyrone | PKSN | alt5 from Alternaria solani | 2005,Fujii et al.An iterative type I polyketide synthase PKSN catalyzes synthesis of the decaketide alternapyrone with regio-specific octa-methylation.Chem Biol. | decaketide-derived a-pyrone with eight methyl branches | a-amylase promoter/A. oryzae | 20 | 10 | 
| |
| Alternaric Acid | Alternaria solani, Alternaria alternata (formerly known as A. kikuchiana) | Herbert1989 | biosynthesized from two polyketide chains. Contribute to disease development in the plant host by the fungus. Condensation if a hexaketide-derived acyl derivative with dihyfrotriacetic acid lactone. | 17 | 6 | 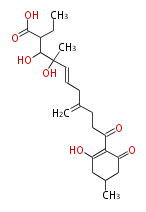
| |||
| Alternariol | Alternaria tenuis | Herbert1989 | 14 | 7 | 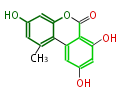
| ||||
| Amphotericin B | Dewick2009 | 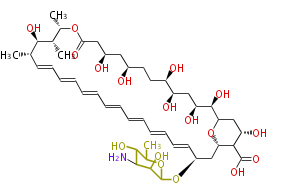
| |||||||
| Andrimid | 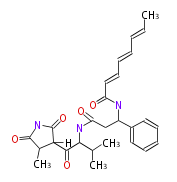
| ||||||||
| Aschochitin | Herbert1989 | structurally related to citrinin and and its biosynthesis similar. | File:Aschochitin.Mol.png | ||||||
| Ascomycin | Dewick2009 | FK520 | 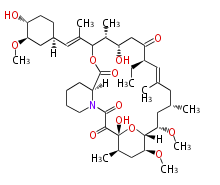
| ||||||
| Aslaniol | PKSF from A. solani | 2009,Fujii.Heterologous expression systems for polyketide synthases.Nat Prod Rep. | dodecaketide | a-amylase promoter/A. oryzae | 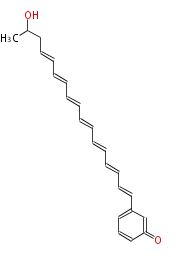
| ||||
| Aslanipyrone | PKSF from A. solani | 2009,Fujii.Heterologous expression systems for polyketide synthases.Nat Prod Rep. | undecaketide | a-amylase promoter/A. oryzae | 
| ||||
| Asperlactone | Aspergillus melleus | Herbert1989 | 8 | 5 | 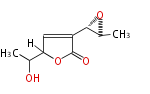
| ||||
| Asperlin | Aspergillus nidulans | Herbert1989 | 8 | 5 | 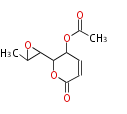
| ||||
| Aspyridone A | Aspergillus nidulans | 2007,Bergmann et al.Genomics-driven discovery of PKS-NRPS hybrid metabolites from Aspergillus nidulans.Nat Chem Biol.;Challis2008 | PKS-NRPS hybrid metabolites | 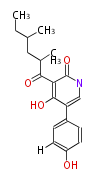
| |||||
| Aspyridone B | Challis2008 | 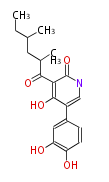
| |||||||
| Aspyrone | Aspergillus melleus | Herbert1989 | 7 | 5 | 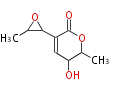
| ||||
| Asteltoxin | Aspergillus stellatus | Herbert1989 ;Kruger,G.J.,Steyn,P.S.,Vleggaar,R.,andRabie,C.J.(1979).X-raycrystalstructureofasteltoxin,anovelmycotoxinfrom Aspergillusstellatus Curzi.J.Chem.Soc.Chem.Commun. 441?442. | linear a-pyrone-containingpolyketide. starter propionate and eight malonate; Or from acetate and methionine instead of propionate. Structurally related to citreoviridin and aurovertin | 9 | 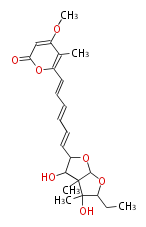
| ||||
| Astepyrone | Aspergillus terreus | Herbert1989 | 4 | 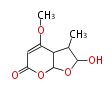
| |||||
| Atrochrysone | Penicillium sp, Aspergillus sp | Dewick2009; | 15 | 8 | 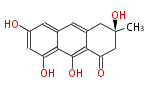
| ||||
| Atrochrysone Aarboxylic Acid | Penicillium sp, Aspergillus sp | Dewick2009; | 16 | 8 | File:Atrochrysone Aarboxylic Acid.Mol.png | ||||
| Aureothin | Streptomyces thioluteus | Herbert1989 | mixed origins, from propionate and single acetate plus p-nitrobenzoic acid | 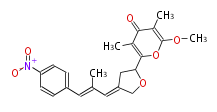
| |||||
| Aurovertin | Streptomyces thioluteus | File:Aurovertin.Mol.png | |||||||
| Austdiol | Herbert1989 ;1983J. Chem. Soc., Chem. Commun_Evidence for a mono-oxygenase mechanism in the biosynthesis of austdiol | Austdiol has a structure similar to citrinin and its biosynthesis appears to be similar. | 5 | 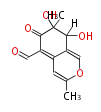
| |||||
| Avenaciolide | Herbert1989 | from acetate/malonate plus succinyl CoA | 
| ||||||
| Averantin | Nor-1;StcE | Aspergillus parasiticus, Aspergillus flavus | SanchezEtal2008;Dewick2009 | Anthraquinone. Hexanoate is the starter unit. Intermediate of Aflatoxin B and sterigmatocystin. | 20 | 10 | 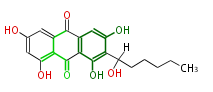
| ||
| Avermectin B1a | Dewick2009 | macrolides | 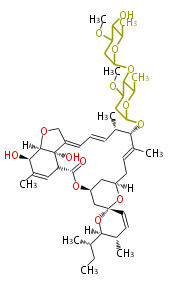
| ||||||
| Avermectin B2a | Dewick2009 | macrolides | 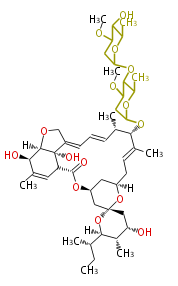
| ||||||
| Averufin | Fas1,Fas2,PksA,Nor-1,AvnA,AdhA | Aspergillus parasiticus, Aspergillus flavus | SanchezEtal2008;Dewick2009 | Hexanoate is the starter unit. Intermediate of Aflatoxin B and sterigmatocystin. | 20 | 10 | 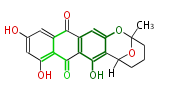
| ||
| Avilamycin | Dewick2009 | 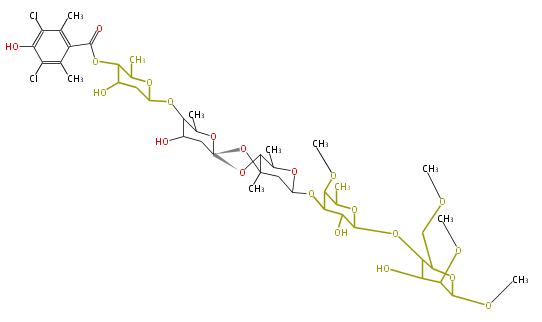
| |||||||
| Barnol | Penicillium baarnense | Herbert1989 | one methyl group derives from methionine and the other by reduction of a carboxy-group. The ethyl group derives form C-2 of acetate and methionine. | 9 | 4 | 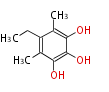
| |||
| Benzophenone | Penicillium griseofulvin | Dewick2009;Herbert1989 | probable intermediate of griseofulvin | 14 | 7 | 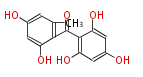
| |||
| Bikaverin | Bikaverin nonaketide synthase | PKS4 from Gibberella fujikuroi | Gibberella fujikuroi | Herbert1989 | nonaketide, from singal chain | E.coli. | 18 | 9 | 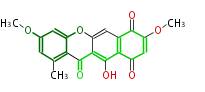
|
| Botryodiplodin | Herbert1989 | like penicillic acid ,via orsellinic acid but cleavage occurs between C-3 and C-4 instead of C-4 and C-5, C-4 is lost at some stage | 4 | 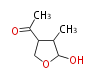
| |||||
| Brefeldin A | Penicillium decumbens, Penicillium brefeldianum, Penicillium cyaneum, Aspergillus clavatus,Eupenicillium brefeldianum, | Herbert1989 | a macrolide, different molecules of oxygen precludes a biosynthetic mechanism similar to prostaglandins. | 16 | 8 | 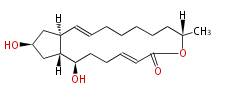
| |||
| Brevetoxin A | Karenia brevis | Dewick2009 | polyether | 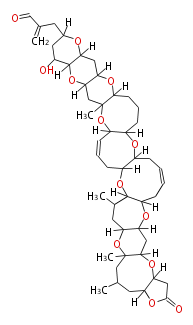
| |||||
| Brevetoxin B | Karenia brevis | Dewick2009 | polyether | 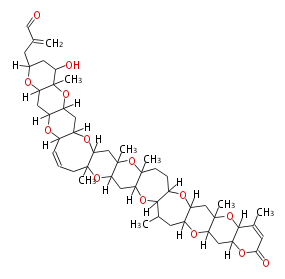
| |||||
| C-1027 | 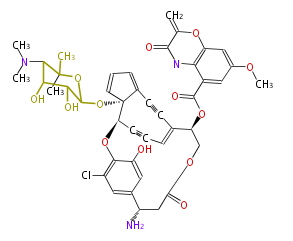
| ||||||||
| Calichemicin Gamma1 | Micromonospora echinospora | 2009,Belecki et al.Production of octaketide polyenes by the calicheamicin polyketide synthase CalE8: implications for the biosynthesis of enediyne core structures.J Am Chem Soc | 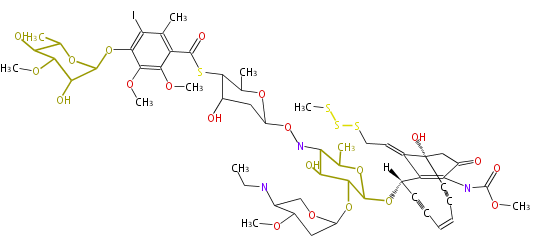
| ||||||
| Cannabidiol | Cannabis sativa (Indian hemp; Cannabaceae) | Dewick2009 | cannabinoids, terpenophenolics, A saturated C6 hexanoate starter unit ,olivetolic acid is the precursor | 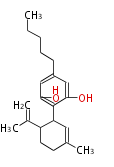
| |||||
| Cannabigerolic Acid | Cannabis sativa (Indian hemp; Cannabaceae) | Dewick2009 | cannabinoids, terpenophenolics, A saturated C6 hexanoate starter unit ,olivetolic acid is the precursor | 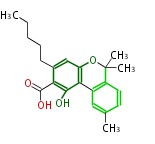
| |||||
| Cannabinol | Cannabis sativa (Indian hemp; Cannabaceae) | Dewick2009 | cannabinoids, terpenophenolics, A saturated C6 hexanoate starter unit ,olivetolic acid is the precursor | 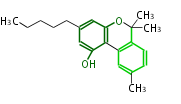
| |||||
| Cercosporin | Cercospora sp, Cercospora kikuchii | Herbert1989 | 7 | 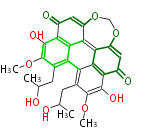
| |||||
| Chlortetracycline | oxy PKS (otc) | OxyD,OxyA,OxyB,OxyC,OxyP,OxyJ,OxyK, OxyJ, OxyN,OxyI,OxyF,OxyL,OxyG,OxyQ,OxyR,OxyT,OxyS,OxyE,TchA | Streptomyces rimosus, Streptomyces aureofaciens | Dewick2009 | starter group is malonamyl-Co. a precursor of tetracycline. Tetracyclic backbone | 19 | 8 | 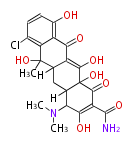
| |
| Chrysomycin A | Streptomyces sp | Herbert1989 | 17 | 9 | 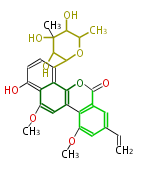
| ||||
| Chrysomycin B | Streptomyces sp | Herbert1989 | 17 | 9 | 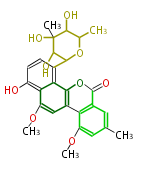
| ||||
| Chrysophanol | Asahina chrysantha, Cassia angustifolia | Dewick2009; | 15 | 8 | 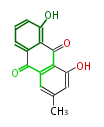
| ||||
| Chrysophanol Anthrone | Asahina chrysantha, Cassia angustifolia | Dewick2009; | 15 | 8 | 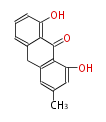
| ||||
| Ciguatoxin I | Gymnothorax javanicus, Lutjanus bohar | Dewick2009 | polyether | 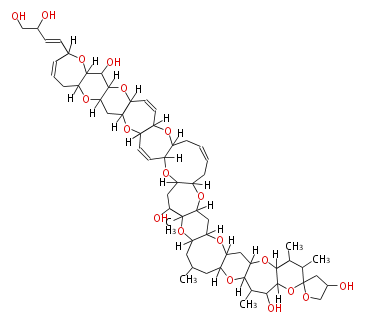
| |||||
| Citreomontanin | Penicillium pedemontanum | 1981,Sylvie Rebuffat, Daniel Davoust and Darius Molho, Phytochemistry, Biosynthesis of citreomontanin in Penicillium pedemontanum | linear a-pyrone-containingpolyketide | 
| |||||
| Citreoviridin | Sakabe,N.,Goto,T.,andHirata,Y.(1977).Structureofcitreoviridin,amycotoxinproducedby Penicilliumcitreo-viride molded onrice.Tetrahedron 33,3077?3081.;Niwa,M.,Endo,T.,Ogiso,S.,Furukawa,H.,andYamamura,S. (1981).Twonewpyrones,metabolitesof Penicilliumcitreo-viride Biouge.Chem.Lett.(Jpn)1285?1288 | linear a-pyrone-containingpolyketide | 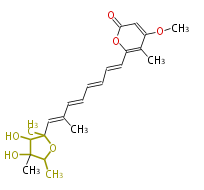
| ||||||
| Citrinin | Penicillium citrinum | Herbert1989 | via a dihydro-isocoumarins | 10 | 5 | 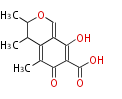
| |||
| Clavatol | Aspergillus clavatus | Herbert1989 | 8 | 4 | 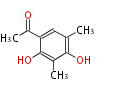
| ||||
| Coarctatin | Herbert1989 | two carbons from methionine | 8 | 4 | 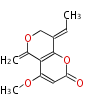
| ||||
| Colletodiol | Cytospora spp | Herbert1989 ;1993Biosynthesis of colletodiol and related polyketide macrodiolides in Cytospora sp. ATCC 20502 : synthesis and metabolism of advanced intermediates | non-aromatic,by tetraketide and triketide | 8 | 7(4+3) | 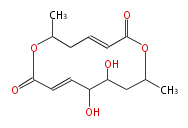
| |||
| Compactin | 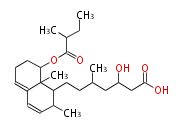
| ||||||||
| Curvularin | Penicillium sp FP1768, Penicillium baradicum Hellllinrll Osporiulll | Herbert1989 | similar in chemical structure to zearalenone | 16 | 8 | 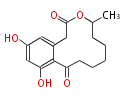
| |||
| Deoxyherquienone | Herbert1989 | fungal phenalenones | 15 | 7 | 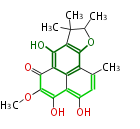
| ||||
| Deoxyradicin | Herbert1989 | 11 | 6 | 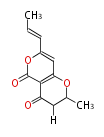
| |||||
| Dihydroisocoumarin | Aspergillus terreus | Herbert1989 | 10 | 5 | 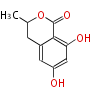
| ||||
| Diplosporin | Diplodia macrospora | Herbert1989 ;1983, Charles P. Gorst-Allman, Pieter S. Steyn and Robert Vleggaar, Biosynthesis of diplosporin by Diplodia macrospora. Part 2. Investigation of ring formation using stable isotopes. J. Chem. Soc., Perkin Trans. 1,?1357 - 1359 | two C1 units from methionine, C11 plus C12 constitute the starter acetate | 10 | 5 | 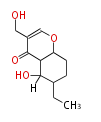
| |||
| Discodermolide | Dewick2009 | 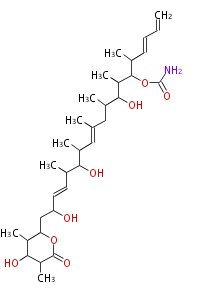
| |||||||
| Doramectin | Dewick2009 | macrolides | 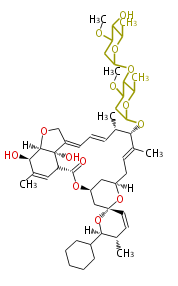
| ||||||
| Dothistromin | 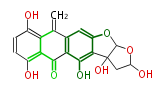
| ||||||||
| Doxorubicin | Streptomyces peuceticus | Dewick2009 | Adriamycin. anthracycline antibiotics,The starter group for the type II PKS is propionyl-CoA | 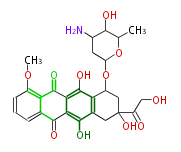
| |||||
| Emodin | Penicillium sp, Rhamnus sp, Rumex sp, Cassia angustifolia | Dewick2009; | 15 | 8 | 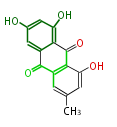
| ||||
| Emodin Anthrone | Penicillium sp, Rhamnus sp, Rumex sp, Cassia angustifolia | Dewick2009; | 15 | 8 | 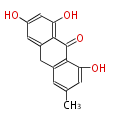
| ||||
| Emodin Dianthrone | Dewick2009 | 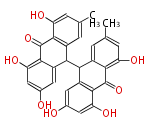
| |||||||
| Endocrocin | Penicillium sp, Rhamnus sp, Rumex sp, Cassia angustifolia | Dewick2009; | 16 | 8 | 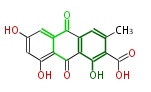
| ||||
| Endocrocin Anthrone | Penicillium sp, Rhamnus sp, Rumex sp, Cassia angustifolia | Dewick2009; | 16 | 8 | 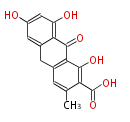
| ||||
| Enniatin B | 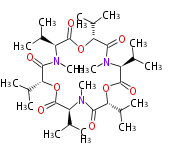
| ||||||||
| Enterocin | Streptomyces maritimus | 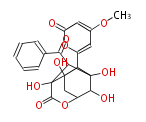
| |||||||
| Epothilone A | Dewick2009 | 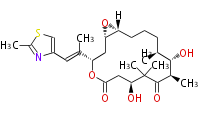
| |||||||
| Epothilone B | Dewick2009 | 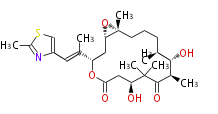
| |||||||
| Epothilone C | Dewick2009 | 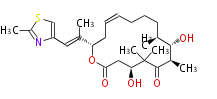
| |||||||
| Epothilone D | Dewick2009 | 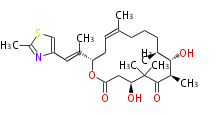
| |||||||
| Epoxydon | Phyllosticta sp | Herbert1989 ;1975 Biosynthesis of Epoxydon and Related Compounds by Phyllosticta sp. Agric. Bioi. Chem. ( Japan), 39,409-13 | 7 | 4 | 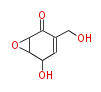
| ||||
| Epsilon-Pyrromycinone | Herbert1989 | 9 acetate units. propionate starter unit is extended by malonate unit. | 20 | 9 | 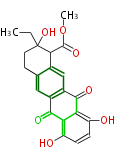
| ||||
| Erythromycin | Dewick2009 | 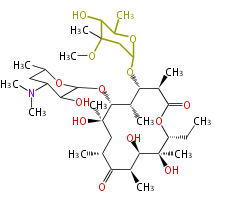
| |||||||
| Flavipin | Aspergillus flaviceps | Herbert1989 | 8 | 4 | 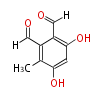
| ||||
| Fumigatin | Aspergillus fumigatus | Herbert1989 | carboxy-group is lost after introduction of the C-5 hydroxy-group;otherwise a symmetrical intermediate would have been generated. | 7 | 4 | 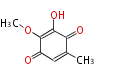
| |||
| Fumonisin B1 | FUM5 | Fusarium verticillioides, Gibberella fujikuroi | Nonaketide | 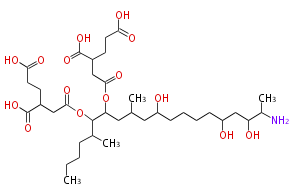
| |||||
| Furanomycin | Herbert1989 | propionate and two acetate | 7 | 3 | 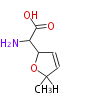
| ||||
| Geldanamycin | 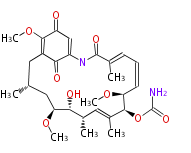
| ||||||||
| Griseofulvin | Penicillium griseofulvin | Dewick2009;Herbert1989 | acetate-derived metabolite,Phenolic oxidative coupling | 14 | 7 | 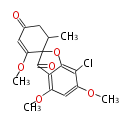
| |||
| Griseophenone B | Penicillium griseofulvin | Dewick2009;Herbert1989 | 14 | 7 | 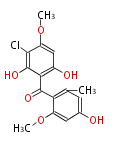
| ||||
| Griseophenone C | Penicillium griseofulvin | Dewick2009;Herbert1989 | 14 | 7 | 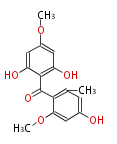
| ||||
| Halichondrin B | Halichondria okadai | Dewick2009 | polyethers, macrolides | 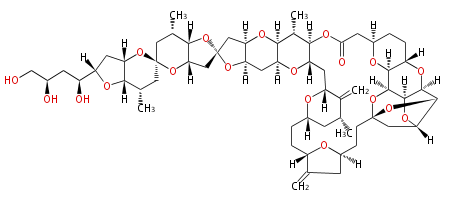
| |||||
| Humulone | Humulus lululus (hop; Cannabaceae) | Dewick2009 | typical bitter taste of beer, foam-stabilizing and antibacterial properties. Formed by oxidative transformation of deoxyhumulone. the starter unit for the polyketide is leucine-derived isovaleryl-CoA. | 13 | 3 | 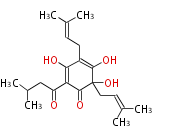
| |||
| Hyperforin | Hypericum perforatum | Dewick2009 | Antidepressive agent in St John’s Wort Polyketide nature is almost entirely obscured by the added isoprenoid fragments | 17 | 3 | 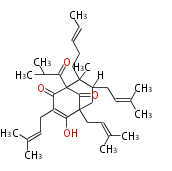
| |||
| Hypericin | Dermocybe spp | Dewick2009 | a constituent of St John’s Wort, Hypericum perforatum (Guttiferae/Hypericaceae). | 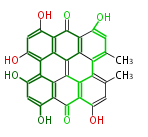
| |||||
| Islandicin | Penicillium islandicum | Dewick2009;Herbert1989 | anthraquinone. Islandicin is another anthraquinone pigment produced by Penicillium islandicum, and differs from emodin in two ways: one hydroxyl is missing and a new hydroxyl has been in corporated adjacent to the methyl. | 15 | 8 | 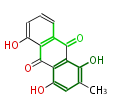
| |||
| Isoepoxydon | Penicillium patulum (=Penicillium urticae) | 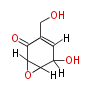
| |||||||
| Isousnic Acid | Usnea spp, Cladonia spp | Dewick2009;Herbert1989 | Incorporated two molecules of methylphloracetophenone by an oxidative coupling mechanism Different from usnic acid. using the alternative hydroxyl nucleophile in heterocyclic ring formation | 14 | 4 | 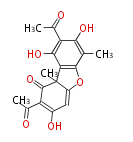
| |||
| Ivermectin | Dewick2009 | macrolides | 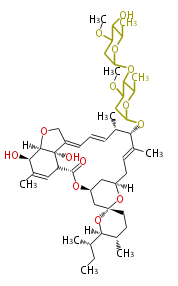
| ||||||
| Khellin | Pentaketide chromone synthase | Ammi visnaga | Dewick2009 | furochromones,aromatic pentaketide.The starter unitis derived by decarboxylation.PCS,Pentaketide chromone synthase, plant-speci?c type III PKS | Escherichia coli | 11 | 5 | 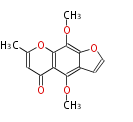
| |
| Lasalocid | Streptomyces lasaliensis | Dewick2009 | polyether antibiotics | 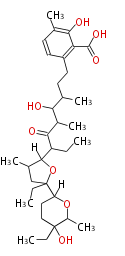
| |||||
| Lecanoric Acid | Umbilicaria arctica, Umbilicaria nylanderiana | Dewick2009;Herbert1989 | A depside (an ester formed from two phenolic acids). Combination of two orsellinic acid thioester molecule | 8 | 4 | 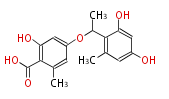
| |||
| Leucomycin A1 | Herbert1989 | 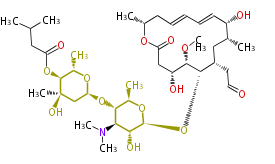
| |||||||
| LL-D253alpha | Phoma pigmentivora | Herbert1989 | chromanone, from two polyketide chain | 11 | 6 | 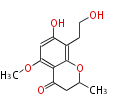
| |||
| Lovastatin | LOVB=LNKS, LovF=LDKS | loVF | Aspergillus terreus | Nonaketide.(mevinolin; monacolin K) | yeast | 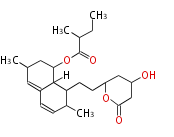
| |||
| M-Cresol | Aspergillus fumigatus | Dewick2009 | methylphenol;3-Hydroxytoluene;3-cresol; derivative of 6-MSA | 7 | 4 | 
| |||
| Melanin | ALB1=PKSP,PKS1 | Aspergillus fumigatus, Nodulisporium sp | Heptaketide | 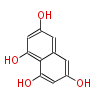
| |||||
| Mellein | Aspergillus melleus, Aspergillus ochraceus | Herbert1989 ;2003_Natural Products_ the Secondary Metabolites (Tutorial Chemistry Texts) | 10 | 5 | 
| ||||
| Methylphloracetophenone | Usnea spp, Cladonia spp | Dewick2009;Herbert1989 | Two molecules of methylphloracetophenone: usnic acid | 8 | 4 | 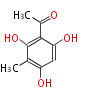
| |||
| Mollisin | Mollisia caesia | Herbert1989 | naphthoquinone.Two potential routes for the biosynthesis of mollisin: one chain and two chains | 11 | 8(8,3+5) | 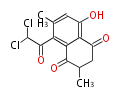
| |||
| Monensin A | Streptomyces cinnamonensis | Herbert1989 | C31H52O9(R1)(R2),Monensin A (R1= -CH(CH3)COOH, R2= -CH2CH3) Monensin B (R1= -CH(CH3)COOH, R2= -CH3) Monensin C (R1= -(CH2)3COOH, R2= -CH2CH3) | 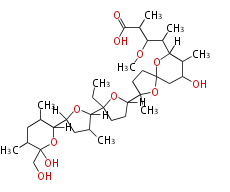
| |||||
| Multicolic Acid | Penicillium multicolor | Herbert1989 ;1998,Application of Isotopic Methods to Secondary Metabolic Pathways. Thomas J.Simpson. Topics in Current Chemistry,Vol.195 | fragmanted polyketide, The arrangement shown requires C-4,5 cleavae before loss of the carboxy-group. | 10 | 6 | 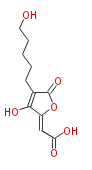
| |||
| Mycolactone | Mycobacterium ulcerans | 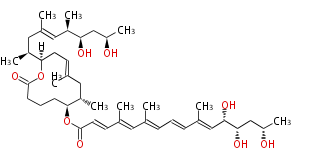
| |||||||
| Mycophenolic Acid | Penicillium brevicompactum | Dewick2009;Herbert1989 | Immunosuppressive agent. 5-methylorsellinic acid is precursor. Addition of farnesyl alkyl. The chain length of the farnesyl alkyl group is subsequently shortened by oxidation of a double bond, giving demethylmycophenolic acid, which is then O-methylated, again involving SAM, to produce mycophenolic acid | 12 | 4 | 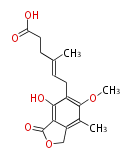
| |||
| Myxalamide | MxaC-1 | Stigmatella aurantiaca | 2007,Pulsawat et al.Characterization of biosynthetic gene cluster for the production of virginiamycin M, a streptogramin type A antibiotic, in Streptomyces virginiae.Gene. | 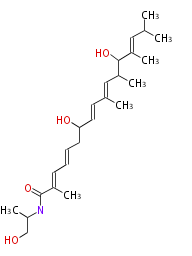
| |||||
| Myxothiazol A | Stigmatella aurantiaca | 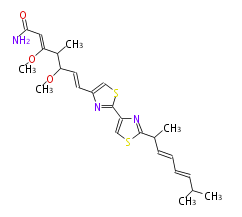
| |||||||
| Myxothiazol Z | Stigmatella aurantiaca | 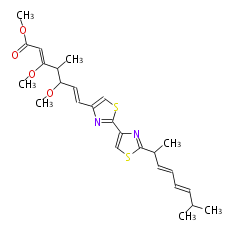
| |||||||
| Niddamycin | Streptomyces caelestis | 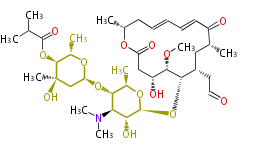
| |||||||
| Norsolorinic Acid | norsolorinic acid(NA) PKS | A?A/B(A.parasiticus); StcJ/K(A.nidulans) | Aspergillus parasiticus, Aspergillus flavus | SanchezEtal2008;Dewick2009 | Anthraquinone. Hexanoate is the starter unit. Intermediate of Aflatoxin B and sterigmatocystin. | 20 | 10 | 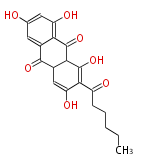
| |
| Nystatin | Streptomyces noursei | Dewick2009 | macrolide,(NYS; Mycostatin (TN)) | 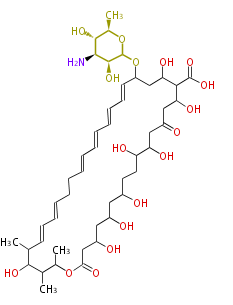
| |||||
| Ochratoxin A | otapks of A. ochraceus; otapksPN gene from P. nordicum; ACpks from A. carbonarius | Aspergillus ochraceus, Penicillium viridicatum,Penicillium verrucosum, Penicillium nordicum, Aspergillus fumigatus, Aspergillus carbonarius | 1979,Huff and Hamilton, W.E. Huff and P.B. Hamilton, Mycotoxins?their biosynthesis in fungi: ochratoxins?metabolites of combined pathways, Journal of Food Protection 42 (1979), pp. 815?820.;2001Harris and Mantle, J.P. Harris and P.G. Mantle, Biosynthesis of ochratoxins by Aspergillus ochraceus, Phytochemistry 58 (2001), pp. 709?716;2009,Gallo et al.Characterisation of a pks gene which is expressed during ochratoxin A production by Aspergillus carbonarius.Int J Food Microbiol. | OTA is a potent nephrotoxin and a possible human carcinogen with a polyketide derived structure.Structurally OTA consists of a polyketide which is believed to be derived from a dihydroiso-coumarin group that is amide-linked to the amino acid L-phenylalanine.Its biosynthesis pathway has yet not been completely elucidated, although a number of putative pathways have been proposed (Harris and Mantle, 2001; Huff and Hamilton, 1979). | 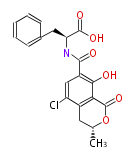
| ||||
| Ochrephilone | Penicillium multicolor | Herbert1989 | from two chains. formed by the condensation of two acetate-derived chains and the introduction of a C, unit (presumably from methionine) at C(4). | 17 | 10(8+2) | 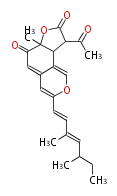
| |||
| Okadaic Acid | Dinophysis sp, Prorocentrum lima | Dewick2009 | polyether,PP1, PP2A inhibitor | 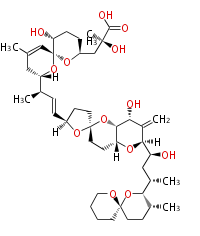
| |||||
| Oleandomycin | Streptomyces antibioticus | Dewick2009 | Amimycin; Landomycin; Matromycin; Romicil | 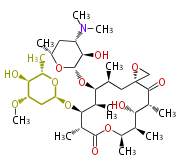
| |||||
| Olivetolic Acid | Cannabis sativa (Indian hemp; Cannabaceae) | Dewick2009 | A saturated C6 hexanoate starter unit | 4 | 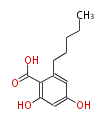
| ||||
| O-Methylasparvenone | Aspergillus parvulus | Herbert1989 ; 1982, Application of?2H?-isotopic shifts in?13C n.m.r. spectra to biosynthetic studies. Incorporation of [1-13C,?2H3]acetate into?O-methylasparvenone in?Aspergillus parvulus. J. Chem. SOC., Chem. Commun.,1074. | formed by the way of naphthalene | 12 | 6 | 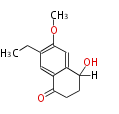
| |||
| Orcinol | Aspergillus fumigatus | Herbert1989 | Collie’s realisation that the triketone might be anintermediate in orcinol biosynthesis was inspirational. synthesized chemically from dehydroacetic acid, likely via a polyketone intermediate,suggested that polyphenols could be biosynthesized from a C2 precursor | 7 | 2 | 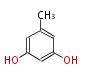
| |||
| Orsellinic Acid | Orsellinic acid synthase (OSAS) | aviM | Streptomyces viridochromogenes, Penicillium madriti | Dewick2009;G.-L. Tang and W. Liu, Biochem. Biophys. Res. Commun., 2006,345, 133?139. | lack1OH group, because formation of conjugated system enolization | 8 | 4 | 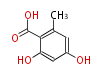
| |
| Oxytetracycline | oxy PKS (otc) | OxyD,OxyA,OxyB,OxyC,OxyP,OxyJ,OxyK, OxyJ, OxyN,OxyI,OxyF,OxyL,OxyG,OxyQ,OxyR,OxyT,OxyS,OxyE,TchA | Streptomyces rimosus, Streptomyces aureofaciens | Dewick2009 | starter group is malonamyl-Co. a precursor of tetracycline. Tetracyclic backbone | 19 | 10 | 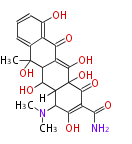
| |
| Palitantin | Penicillium palitans | Herbert1989 | nonaromatic six membered ring. C-12 was unexpectedly not labelled by 18O, but this is the consequence of ready exchange at this position | 14 | 7 | 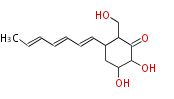
| |||
| Patulin | MSAS;ATX(=MSAS) | Aspergillus clavatus,Aspergillus terreus,Penicillium patulum (=Penicillium urticae) | Dewick2009;1971,Aberhart and Caspi.The fate of the 6 alpha-hydrogen of 5 alpha-cholest-7-en-3 beta-ol in the conversion to 7-dehydrocholesterol by rat liver microsomes.J Biol Chem. | derived from acetate via 6-methylsalicylic acid | 6 | 4 | 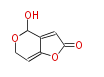
| ||
| Penicillic Acid | Penicillium cyclopium, Penicillium baarnense | Herbert1989; Dewick2009 | This time orsellinic acid is aprecursor, and ring fission appears to proceed viaa quinone, which is the result of decarboxylation,oxidation, and methylation reactions | 6 | 4 | 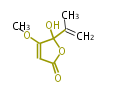
| |||
| Phloracetophenone | Aspergillus clavatus | 8 | 4 | 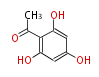
| |||||
| Phlorisobutyrophenone | Isobutyrophenonesynthase(BUS) | Hypericum calycinum | Dewick2009 | analogue of phloroacetophenone. But using isobutyryl-CoA as starter instead of acetyl-CoA. Extended by three malonyl-CoA. type III PKS. using isovalery-CoA as starter instead of acetyl-CoA. Extended by three malonyl-CoA.type III PKS. Precursor of humulone | 3 | 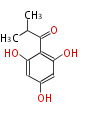
| |||
| Phlorisovalerophenone | phlorisovalerophenone synthase(VPS) | Humulus lululus (hop; Cannabaceae) | Dewick2009 | using isovalery-CoA as starter instead of acetyl-CoA. Extended by three malonyl-CoA.type III PKS. Precursor of humulone | 3 | 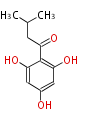
| |||
| Physcion | Penicillium sp, Rhamnus sp, Rumex sp, Cassia angustifolia | Dewick2009; | 15 | 8 | 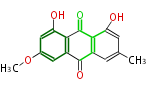
| ||||
| Picromycin | Streptomyces venezuelae | Herbert1989 | 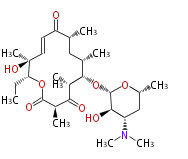
| ||||||
| Pimaricin | 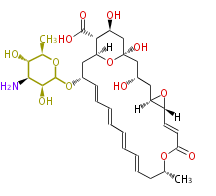
| ||||||||
| Platenomycin | Herbert1989 | The platenomycins are closely related to leucomycin | File:Platenomycin.Mol.png | ||||||
| Pretetramide | oxy PKS (otc) | OxyD,OxyA,OxyB,OxyC,OxyP,OxyJ,OxyK, OxyJ, OxyN,OxyI | Streptomyces rimosus,Streptomyces aureofaciens | Dewick2009 | Malonamyl-CoA is the starter unit.Tetracyclic backbone with all carbon atoms from malonated derived precursor of tetracycline | 19 | 9 | 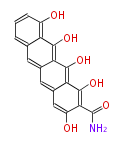
| |
| Protohypericin | Streptomyces natalensis | Dewick2009 | 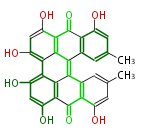
| ||||||
| Pyochelin | Pseudomonas aeruginosa | 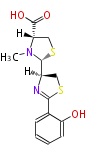
| |||||||
| Pyoluteorin | 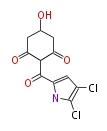
| ||||||||
| Radicin | Herbert1989 | formed from two polyketide chains; alternatively they may be formed by a pathway involving ring-cleavage. | 11 | 6 | 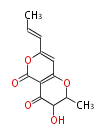
| ||||
| Rapamycin | Streptomyces hygroscopicus | Dewick2009 | 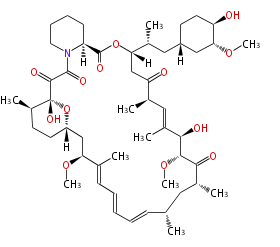
| ||||||
| Ravenilin | Helminthosporium ravenelii, Helminthosporium turcicum | Herbert1989 | xanthone,Islandicin is plausibly an intermediate in ravenilin biosynthesis | 12 | 8 | 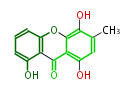
| |||
| Rhein | Cassia angustifolia | 2002,Dewick.Medicinal natural products : a biosynthetic approach.Wiley;Dewick2009; | 15 | 8 | 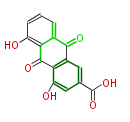
| ||||
| Rifabutin | Dewick2009 | 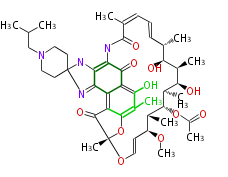
| |||||||
| Rifamycin B | Amycolatopsis mediterranei | Dewick2009 | 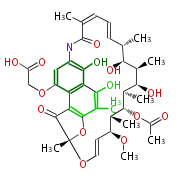
| ||||||
| Rifapentine | Dewick2009 | 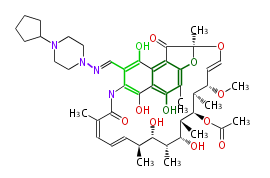
| |||||||
| Rosellisin | Herbert1989 | fungal alpha-pyrones. Two carbons are derived from methionine | 8 | 4 | 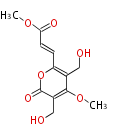
| ||||
| Rubrofusarin | 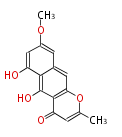
| ||||||||
| Rubropunctatin | Monascus pilosus | Herbert1989 | Rubropunctatin can be split into two fragments on the basis of labelling results. The left-hand part derives in the usual way. The left-habd part -though seems to derive through the beta-keto acid formed by condensation of hexanoic acid with an acetate unit. | 17 | 6 | 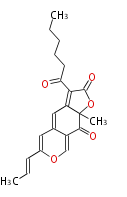
| |||
| Sclerin | Sclerotinia spp | Herbert1989 | unusual, from two polyketide chains | 9 | 5 | 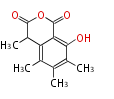
| |||
| Sclerotinin A | Sclerotinia spp | Herbert1989 | from one polyketide chains | 10 | 5 | 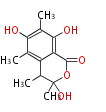
| |||
| SEK4 | octaketide synthase OKS | Escherichia coli | Dewick2009 | A type III octaketide synthase gene from the anthraquinone-producing plant Aloe arborescens, when expressed in Escherichia coli, did not synthesize anthraquinones, but instead the two products SEK4 and SEK4b, not normally found in Aloe | 16 | 8 | 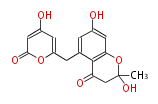
| ||
| SEK4b | octaketide synthase OKS | Escherichia coli | Dewick2009 | A type III octaketide synthase gene from the anthraquinone-producing plant Aloe arborescens, when expressed in Escherichia coli, did not synthesize anthraquinones, but instead the two products SEK4 and SEK4b, not normally found in Aloe | 16 | 8 | 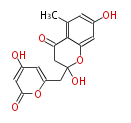
| ||
| Selamectin | Dewick2009 | macrolides | 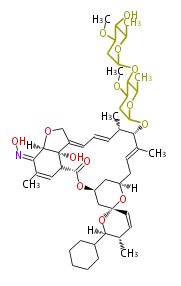
| ||||||
| Sennidin A | Cassia angustifolia, Cassia senna (syn Cassia acutifolia) | Dewick2009 | dianthrone O-glycosides | 8 | 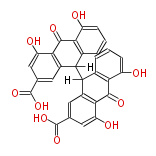
| ||||
| Sennidin B | Cassia angustifolia, Cassia senna (syn Cassia acutifolia) | Dewick2009 | dianthrone O-glycosides | 8 | 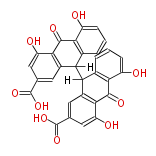
| ||||
| Sennidin C | Cassia angustifolia, Cassia senna (syn Cassia acutifolia) | Dewick2009 | dianthrone O-glycosides | 8 | 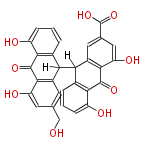
| ||||
| Sennidin D | Cassia angustifolia, Cassia senna (syn Cassia acutifolia) | Dewick2009 | dianthrone O-glycosides | 8 | 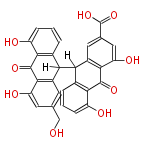
| ||||
| Sennosides A | Cassia angustifolia, Cassia senna (syn Cassia acutifolia) | Dewick2009 | dianthrone O-glycosides | 8 | 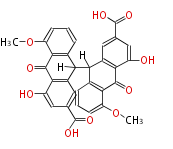
| ||||
| Sennosides B | Cassia angustifolia, Cassia senna (syn Cassia acutifolia) | Dewick2009 | dianthrone O-glycosides | 8 | 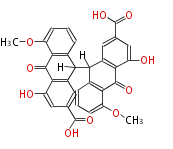
| ||||
| Sennosides C | Cassia angustifolia, Cassia senna (syn Cassia acutifolia) | Dewick2009 | dianthrone O-glycosides | 8 | 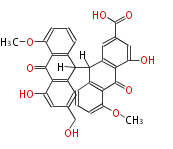
| ||||
| Sennosides D | Cassia angustifolia, Cassia senna (syn Cassia acutifolia) | Dewick2009 | dianthrone O-glycosides | 8 | 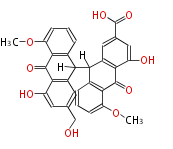
| ||||
| Sepedonin | fungal tropolone | 5 | 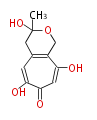
| ||||||
| Siccanin | Helminthosporium siccans | Herbert1989 | Antifungal drug,The fungal metabolite siccanin (21) contains a sesquiterpenoid fragment and a fragment derived from orsellinic acid. | 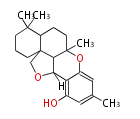
| |||||
| Silvaticamide | Aspergillus silvaticus | Herbert1989 ;1985 CPB_Biosynthesis of Silvaticamide, a Toxin from Aspergillus silvaticus | derived by fragmentation of an anthraquinone, have similar prenylated skeleton with tajixanthone | 17 | 8(12) | 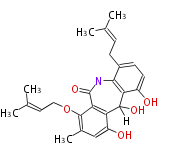
| |||
| SMA76a | pks4 from Gibberlla fujikuroi | Herbert1989 | nonaketide, from singal chain.The PKS4 from Gibberella fujikuroi expressed in E.coli (pET24/BL21(DE3)) showed in vitro activity to form SMA76a 58 from malonyl-CoA, which has the same nonaketide carbon skeleton with bikaverin. If selected octanoyl-CoA over malonyl-CoA as the starter unit and synthesized SMA76b and SMA76C, isocoumarins with acyl and alkyl side-chains, respectively. | E.coli (pET24/BL21(DE3)) | 18 | 9 | 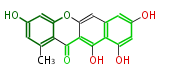
| ||
| SMA76b | pks4 from Gibberlla fujikuroi | Herbert1989 | nonaketide, from singal chain.The PKS4 from Gibberella fujikuroi expressed in E.coli (pET24/BL21(DE3)) showed in vitro activity to form SMA76a 58 from malonyl-CoA, which has the same nonaketide carbon skeleton with bikaverin. If selected octanoyl-CoA over malonyl-CoA as the starter unit and synthesized SMA76b and SMA76C, isocoumarins with acyl and alkyl side-chains, respectively. | E.coli (pET24/BL21(DE3)) | 9 | 
| |||
| SMA76c | pks4 from Gibberlla fujikuroi | Herbert1989 | nonaketide, from singal chain.The PKS4 from Gibberella fujikuroi expressed in E.coli (pET24/BL21(DE3)) showed in vitro activity to form SMA76a 58 from malonyl-CoA, which has the same nonaketide carbon skeleton with bikaverin. If selected octanoyl-CoA over malonyl-CoA as the starter unit and synthesized SMA76b and SMA76C, isocoumarins with acyl and alkyl side-chains, respectively. | E.coli (pET24/BL21(DE3)) | 9 | 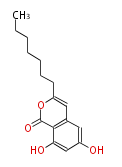
| |||
| Soraphen A | Sorangium cellulosum | 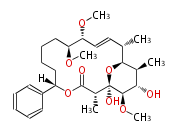
| |||||||
| Spinulosin | Aspergillus fumigatus | 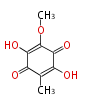
| |||||||
| Spiramycin I | 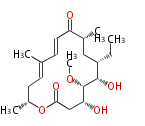
| ||||||||
| Sterigmatocystin | NSAS,PKS-st(STCA) | Aspergillus nidulans(pksST)(stcA)(Fas1,Fas2,PksA,Nor-1,AvnA, AdhA, AvfA, EstA,VBS, VerB,AflN,AflM,OmtB) | Aspergillus parasiticus, Aspergillus flavus, Aspergillus nidulans | Yabe&Nakajima2004;Dewick2009;2009,Hertweck.The biosynthetic logic of polyketide diversity.Angew Chem Int Ed Engl. | Norsolorinic acid and averufin are its precursors Hexanoate is the starter unit. Intermediate of Aflatoxin B and sterigmatocystin. | 15 | 10 | 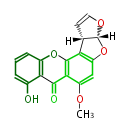
| |
| Stigmatellin A | Stigmatella aurantiaca | 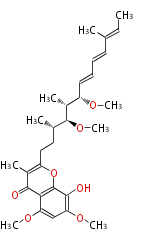
| |||||||
| Stipitatonic Acid | Penicillium stipitatum | Herbert1989 ;1963,Bentley.Biosynthesis of tropolones in Penicillium stipitatum.” J Biol Chem. | 3-methylorsellinic acid is a intermediate, tropolone containing metabolite | 9 | 4 | 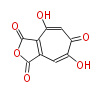
| |||
| Sulochrin | Aspergillus terreus, Aspergillus fumigatus | Herbert1989 | via the anthraquinone. fungal p-methylbenzophenone derivatives | 13 | 8 | 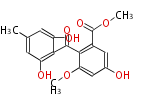
| |||
| Tacrolimus | Streptomyces sp | Dewick2009 | 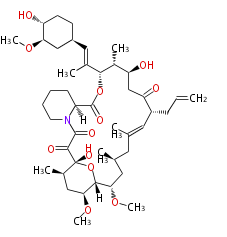
| ||||||
| Tajixanthone | Aspergillus variecolor | Herbert1989 | derived by fragmentation of an anthraquinone, have similar prenylated skeleton with silvaticamide | 20 | 8(12) | 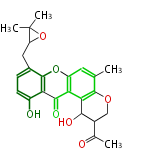
| |||
| Tennellin | fungal PKS-NRPS | a-amylase promoter/A. oryzae | 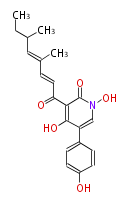
| ||||||
| Terrein | Aspergillus terreus, Aspergillus fumigatus | Herbert1989 | via a dihydro-isocoumarins; biosynthesis pathway is incompleted | 8 | 5 | 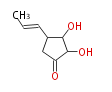
| |||
| Terrequinone A | Aspergillus nidulans | 2007,Corre and Challis.Heavy tools for genome mining.Chem Biol.;[8]2007,Bouhired et al.Accurate prediction of the Aspergillus nidulans terrequinone gene cluster boundaries using the transcriptional regulator LaeA.Fungal Genet Biol. | cryptic NRPS system | TdiA-E in E. coli | 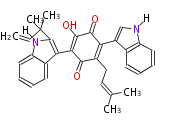
| ||||
| Tetracenomycin F2 | tcm | Streptomyces glaucescens | 2008,Ames et al.Crystal structure and functional analysis of tetracenomycin ARO/CYC: implications for cyclization specificity of aromatic polyketides.Proc Natl Acad Sci U S A. | type II polyketide products | 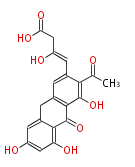
| ||||
| Tetracycline | oxy PKS (otc) | OxyD,OxyA,OxyB,OxyC,OxyP,OxyJ,OxyK, OxyJ, OxyN,OxyI,OxyF,OxyL,OxyG,OxyQ,OxyR,OxyT,OxyS,OxyE,TchA | Streptomyces rimosus,Streptomyces aureofaciens | Dewick2009 | starter group is malonamyl-Co. a precursor of tetracycline. Tetracyclic backbone | 19 | 9 | 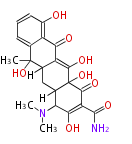
| |
| Tetrahydro Cannabinol | Cannabis sativa (Indian hemp; Cannabaceae) | Dewick2009 | Dronabinol; delta9-Tetrahydrocannabinol; Tetrahydrocannabinol.A saturated C6 hexanoate starter unit The aromatic ring/C5 chain originates from hexanoate and malonate, then cyclization.Precursor isolivetolic acid | 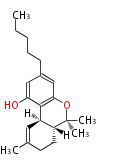
| |||||
| Tetrahydroxynaphthalene | tetrahydroxynaphthalene synthase (THNS);RppA | pks1, from Colletotrichum lagenarium | Streptomyces griseus, Streptomyces coelicolor, Colletotrichum lagenarium | 2001,Pfeifer and Khosla.Biosynthesis of polyketides in heterologous hosts.Microbiol Mol Biol Rev.;2007,American Chemical Society. Meeting (229th : 2005 : San Diego Calif.) et al.Polyketides : biosynthesis, biological activity, and genetic engineering.American Chemical Society : Distributed by Oxford University Press | intermediate of napyradiomycin A | Aspergillus oryzae | 10 | 5 | 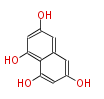
|
| Triacetic Acid Lactone | 2-pyrone synthase | Gerbera hybrida | 2005BB_Microbial Synthesis of Triacetic Acid Lactone | Ether ring formation by cyclization of the 3,5-diketohexanoate thioester ;not only one pks can produce TAL | Escherichia coli and Saccharomyces cerevisiae | 6 | 3 | 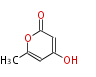
| |
| Trypacidin | Aspergillus fumigatus | 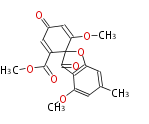
| |||||||
| T-Toxin | PKS1 | Cochliobolus heterostrophus | 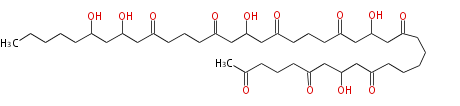
| ||||||
| Tylactone | Streptomyces fradiae | 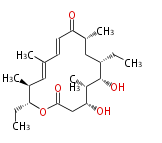
| |||||||
| Tylosin | Streptomyces fradiae | Dewick2009 | 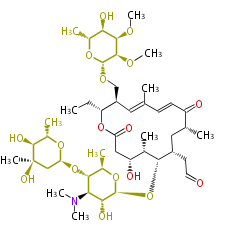
| ||||||
| Urushiol III | Toxicodendron radicans,Toxicodendron toxicaria | Dewick2009 | 3-(8,11-Pentadecadienyl)-1,2-benzenediol. palmitoleoyl-CoA ( 9-hexadecenoyl-CoA) can act as starter group for extension by three malonyl-CoA units | 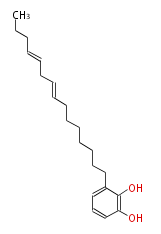
| |||||
| Usnic Acid | Usnea spp, Cladonia spp, Flavocetraria cucculata, Flavocetraria nivalis | Dewick2009; | antibacterial metabolite.formed by two methylphloracetophenone. Incorporated two molecules of methylphloracetophenone by an oxidative coupling mechanism. c-methylation by SAM | 14(8,8) | 8(4,4) | 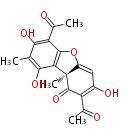
| |||
| Variotin | Herbert1989 | from acetate(malonate) and methionine with the pyrrolidone ring having its origins in glutamic acid | 12 | 6 | 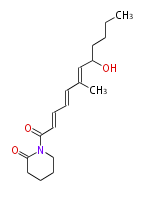
| ||||
| Versicolorin A | Fas1,Fas2,PksA,Nor-1,AvnA, AdhA, AvfA, EstA,VBS, VerB | Aspergillus parasiticus, Aspergillus flavus | Yabe&Nakajima2004;Dewick2009 | Norsolorinic acid and averufin are its precursors Hexanoate is the starter unit. Intermediate of Aflatoxin B and sterigmatocystin. | 17 | 10 | 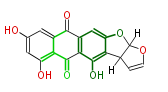
| ||
| Versicolorin B | Fas1,Fas2,PksA,Nor-1,AvnA, AdhA, AvfA, EstA,VBS | Aspergillus parasiticus, Aspergillus flavus | Yabe&Nakajima2004;Dewick2009 | Norsolorinic acid and averufin are its precursors Hexanoate is the starter unit. Intermediate of Aflatoxin B and sterigmatocystin. | 17 | 10 | 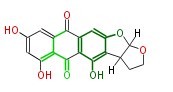
| ||
| Versiconal | Fas1,Fas2,PksA,Nor-1,AvnA,AdhA,AvfA,EstA | Aspergillus parasiticus, Aspergillus flavus | Yabe&Nakajima2004;Dewick2009 | Norsolorinic acid and averufin are its precursors Hexanoate is the starter unit. Intermediate of Aflatoxin B and sterigmatocystin. | 17 | 10 | | ||
| Versiconal Acetate | Fas1,Fas2,PksA,Nor-1,AvnA,AdhA,AvfA | Aspergillus parasiticus, Aspergillus flavus | Yabe&Nakajima2004;Dewick2009 | Norsolorinic acid and averufin are its precursors Hexanoate is the starter unit. Intermediate of Aflatoxin B and sterigmatocystin. | 17 | 10 | | ||
| Visnagin | Pentaketide chromone synthase | Ammi visnaga | Dewick2009 | furochromones,aromatic pentaketide.The starter unitis derived by decarboxylation.precursor of the well known antiasthmatic furochromones, kehellin.PCS,Pentaketide chromone synthase ,plant-speci?c type III PKS | Escherichia coli | 11 | 5 | 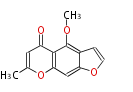
| |
| WA-Naphthopyron | wA | Aspergillus nidulans | 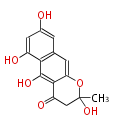
| ||||||
| Zaragozic Acids A | Sporomiella intermedia, Leptodontium elatius | 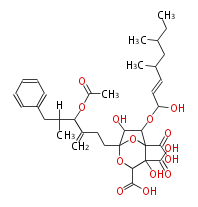
| |||||||
| Zearalenone | Dewick2009 | 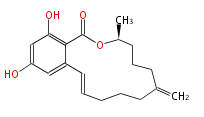
|