Mol:FL3FFCGS0013
From Metabolomics.JP
(Difference between revisions)
| Line 1: | Line 1: | ||
| − | + | ||
| − | + | ||
| − | Copyright: ARM project http://www.metabolome.jp/ | + | Copyright: ARM project http://www.metabolome.jp/ |
| − | 45 49 0 0 0 0 0 0 0 0999 V2000 | + | 45 49 0 0 0 0 0 0 0 0999 V2000 |
| − | -0.1377 0.5700 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -0.1377 0.5700 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -0.1377 0.0079 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -0.1377 0.0079 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 0.3134 -0.2525 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 0.3134 -0.2525 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 0.7645 0.0079 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 0.7645 0.0079 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 0.7645 0.5287 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 0.7645 0.5287 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 0.3134 0.7892 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 0.3134 0.7892 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 1.2155 -0.2525 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 1.2155 -0.2525 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 1.6666 0.0079 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 1.6666 0.0079 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 1.6666 0.5287 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 1.6666 0.5287 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 1.2155 0.7892 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | 1.2155 0.7892 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 1.2155 -0.6586 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | 1.2155 -0.6586 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 2.1175 0.7891 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 2.1175 0.7891 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 2.5772 0.5236 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 2.5772 0.5236 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 3.0369 0.7891 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 3.0369 0.7891 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 3.0369 1.3199 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 3.0369 1.3199 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 2.5772 1.5853 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 2.5772 1.5853 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 2.1175 1.3199 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 2.1175 1.3199 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 0.3134 -0.7723 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | 0.3134 -0.7723 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -0.5905 0.8689 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | -0.5905 0.8689 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 0.3134 1.3676 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | 0.3134 1.3676 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 2.5651 2.2456 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | 2.5651 2.2456 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -3.3072 0.7159 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -3.3072 0.7159 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -2.7916 0.0352 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -2.7916 0.0352 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -2.0491 0.3240 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -2.0491 0.3240 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -1.3327 0.3317 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -1.3327 0.3317 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -1.8533 0.8525 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | -1.8533 0.8525 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -2.6117 0.5801 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -2.6117 0.5801 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -3.8837 0.3830 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | -3.8837 0.3830 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -3.5954 -0.0039 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | -3.5954 -0.0039 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -1.6237 -0.3902 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | -1.6237 -0.3902 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -4.3442 -1.7093 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | -4.3442 -1.7093 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -3.5547 -1.1198 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -3.5547 -1.1198 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -3.0391 -1.8004 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -3.0391 -1.8004 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -2.2966 -1.5116 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -2.2966 -1.5116 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -1.5802 -1.5039 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -1.5802 -1.5039 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -2.1008 -0.9832 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | -2.1008 -0.9832 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -2.8592 -1.2555 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -2.8592 -1.2555 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -3.0391 -2.2456 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | -3.0391 -2.2456 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -1.8712 -2.2258 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | -1.8712 -2.2258 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 3.6298 1.7495 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | 3.6298 1.7495 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 4.3442 1.3370 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 4.3442 1.3370 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -2.9073 1.3875 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -2.9073 1.3875 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -2.1928 0.9750 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | -2.1928 0.9750 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -3.0416 -0.8571 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -3.0416 -0.8571 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -3.8385 -0.6435 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | -3.8385 -0.6435 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 1 2 1 0 0 0 0 | + | 1 2 1 0 0 0 0 |
| − | 2 3 2 0 0 0 0 | + | 2 3 2 0 0 0 0 |
| − | 3 4 1 0 0 0 0 | + | 3 4 1 0 0 0 0 |
| − | 4 5 2 0 0 0 0 | + | 4 5 2 0 0 0 0 |
| − | 5 6 1 0 0 0 0 | + | 5 6 1 0 0 0 0 |
| − | 6 1 2 0 0 0 0 | + | 6 1 2 0 0 0 0 |
| − | 4 7 1 0 0 0 0 | + | 4 7 1 0 0 0 0 |
| − | 7 8 1 0 0 0 0 | + | 7 8 1 0 0 0 0 |
| − | 8 9 2 0 0 0 0 | + | 8 9 2 0 0 0 0 |
| − | 9 10 1 0 0 0 0 | + | 9 10 1 0 0 0 0 |
| − | 10 5 1 0 0 0 0 | + | 10 5 1 0 0 0 0 |
| − | 7 11 2 0 0 0 0 | + | 7 11 2 0 0 0 0 |
| − | 9 12 1 0 0 0 0 | + | 9 12 1 0 0 0 0 |
| − | 12 13 2 0 0 0 0 | + | 12 13 2 0 0 0 0 |
| − | 13 14 1 0 0 0 0 | + | 13 14 1 0 0 0 0 |
| − | 14 15 2 0 0 0 0 | + | 14 15 2 0 0 0 0 |
| − | 15 16 1 0 0 0 0 | + | 15 16 1 0 0 0 0 |
| − | 16 17 2 0 0 0 0 | + | 16 17 2 0 0 0 0 |
| − | 17 12 1 0 0 0 0 | + | 17 12 1 0 0 0 0 |
| − | 3 18 1 0 0 0 0 | + | 3 18 1 0 0 0 0 |
| − | 1 19 1 0 0 0 0 | + | 1 19 1 0 0 0 0 |
| − | 6 20 1 0 0 0 0 | + | 6 20 1 0 0 0 0 |
| − | 16 21 1 0 0 0 0 | + | 16 21 1 0 0 0 0 |
| − | 22 23 1 1 0 0 0 | + | 22 23 1 1 0 0 0 |
| − | 23 24 1 1 0 0 0 | + | 23 24 1 1 0 0 0 |
| − | 25 24 1 1 0 0 0 | + | 25 24 1 1 0 0 0 |
| − | 25 26 1 0 0 0 0 | + | 25 26 1 0 0 0 0 |
| − | 26 27 1 0 0 0 0 | + | 26 27 1 0 0 0 0 |
| − | 27 22 1 0 0 0 0 | + | 27 22 1 0 0 0 0 |
| − | 22 28 1 0 0 0 0 | + | 22 28 1 0 0 0 0 |
| − | 23 29 1 0 0 0 0 | + | 23 29 1 0 0 0 0 |
| − | 24 30 1 0 0 0 0 | + | 24 30 1 0 0 0 0 |
| − | 32 33 1 1 0 0 0 | + | 32 33 1 1 0 0 0 |
| − | 33 34 1 1 0 0 0 | + | 33 34 1 1 0 0 0 |
| − | 35 34 1 1 0 0 0 | + | 35 34 1 1 0 0 0 |
| − | 35 36 1 0 0 0 0 | + | 35 36 1 0 0 0 0 |
| − | 36 37 1 0 0 0 0 | + | 36 37 1 0 0 0 0 |
| − | 37 32 1 0 0 0 0 | + | 37 32 1 0 0 0 0 |
| − | 33 38 1 0 0 0 0 | + | 33 38 1 0 0 0 0 |
| − | 34 39 1 0 0 0 0 | + | 34 39 1 0 0 0 0 |
| − | 31 32 1 0 0 0 0 | + | 31 32 1 0 0 0 0 |
| − | 35 30 1 0 0 0 0 | + | 35 30 1 0 0 0 0 |
| − | 25 19 1 0 0 0 0 | + | 25 19 1 0 0 0 0 |
| − | 15 40 1 0 0 0 0 | + | 15 40 1 0 0 0 0 |
| − | 40 41 1 0 0 0 0 | + | 40 41 1 0 0 0 0 |
| − | 27 42 1 0 0 0 0 | + | 27 42 1 0 0 0 0 |
| − | 42 43 1 0 0 0 0 | + | 42 43 1 0 0 0 0 |
| − | 37 44 1 0 0 0 0 | + | 37 44 1 0 0 0 0 |
| − | 44 45 1 0 0 0 0 | + | 44 45 1 0 0 0 0 |
| − | M STY 1 1 SUP | + | M STY 1 1 SUP |
| − | M SLB 1 1 1 | + | M SLB 1 1 1 |
| − | M SAL 1 2 40 41 | + | M SAL 1 2 40 41 |
| − | M SBL 1 1 44 | + | M SBL 1 1 44 |
| − | M SMT 1 OCH3 | + | M SMT 1 OCH3 |
| − | M SBV 1 44 -4.9815 7.4654 | + | M SBV 1 44 -4.9815 7.4654 |
| − | M STY 1 2 SUP | + | M STY 1 2 SUP |
| − | M SLB 1 2 2 | + | M SLB 1 2 2 |
| − | M SAL 2 2 42 43 | + | M SAL 2 2 42 43 |
| − | M SBL 2 1 46 | + | M SBL 2 1 46 |
| − | M SMT 2 ^CH2OH | + | M SMT 2 ^CH2OH |
| − | M SBV 2 46 -5.8699 7.8431 | + | M SBV 2 46 -5.8699 7.8431 |
| − | M STY 1 3 SUP | + | M STY 1 3 SUP |
| − | M SLB 1 3 3 | + | M SLB 1 3 3 |
| − | M SAL 3 2 44 45 | + | M SAL 3 2 44 45 |
| − | M SBL 3 1 48 | + | M SBL 3 1 48 |
| − | M SMT 3 ^CH2OH | + | M SMT 3 ^CH2OH |
| − | M SBV 3 48 -5.7567 7.4342 | + | M SBV 3 48 -5.7567 7.4342 |
| − | S SKP 8 | + | S SKP 8 |
| − | ID FL3FFCGS0013 | + | ID FL3FFCGS0013 |
| − | KNApSAcK_ID C00004431 | + | KNApSAcK_ID C00004431 |
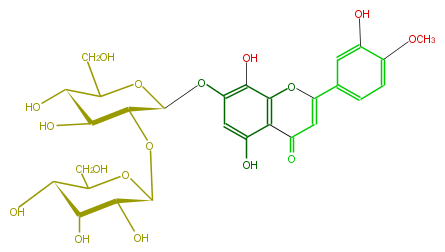
| − | NAME 8-Hydroxyluteolin 4'-methyl ether 7-allosyl-(1->2)-glucoside | + | NAME 8-Hydroxyluteolin 4'-methyl ether 7-allosyl-(1->2)-glucoside |
| − | CAS_RN 114611-04-0 | + | CAS_RN 114611-04-0 |
| − | FORMULA C28H32O17 | + | FORMULA C28H32O17 |
| − | EXACTMASS 640.163949598 | + | EXACTMASS 640.163949598 |
| − | AVERAGEMASS 640.54348 | + | AVERAGEMASS 640.54348 |
| − | SMILES C(CO)(C1O)OC(Oc(c5)c(c(O3)c(c5O)C(C=C(c(c4)ccc(OC)c4O)3)=O)O)C(OC(C2O)OC(C(C2O)O)CO)C1O | + | SMILES C(CO)(C1O)OC(Oc(c5)c(c(O3)c(c5O)C(C=C(c(c4)ccc(OC)c4O)3)=O)O)C(OC(C2O)OC(C(C2O)O)CO)C1O |
M END | M END | ||
| − | |||
Latest revision as of 09:00, 14 March 2009
Copyright: ARM project http://www.metabolome.jp/
45 49 0 0 0 0 0 0 0 0999 V2000
-0.1377 0.5700 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-0.1377 0.0079 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
0.3134 -0.2525 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
0.7645 0.0079 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
0.7645 0.5287 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
0.3134 0.7892 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
1.2155 -0.2525 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
1.6666 0.0079 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
1.6666 0.5287 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
1.2155 0.7892 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
1.2155 -0.6586 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
2.1175 0.7891 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
2.5772 0.5236 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
3.0369 0.7891 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
3.0369 1.3199 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
2.5772 1.5853 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
2.1175 1.3199 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
0.3134 -0.7723 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
-0.5905 0.8689 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
0.3134 1.3676 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
2.5651 2.2456 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
-3.3072 0.7159 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-2.7916 0.0352 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-2.0491 0.3240 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-1.3327 0.3317 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-1.8533 0.8525 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
-2.6117 0.5801 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-3.8837 0.3830 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
-3.5954 -0.0039 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
-1.6237 -0.3902 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
-4.3442 -1.7093 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
-3.5547 -1.1198 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-3.0391 -1.8004 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-2.2966 -1.5116 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-1.5802 -1.5039 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-2.1008 -0.9832 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
-2.8592 -1.2555 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-3.0391 -2.2456 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
-1.8712 -2.2258 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
3.6298 1.7495 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
4.3442 1.3370 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-2.9073 1.3875 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-2.1928 0.9750 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
-3.0416 -0.8571 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-3.8385 -0.6435 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
1 2 1 0 0 0 0
2 3 2 0 0 0 0
3 4 1 0 0 0 0
4 5 2 0 0 0 0
5 6 1 0 0 0 0
6 1 2 0 0 0 0
4 7 1 0 0 0 0
7 8 1 0 0 0 0
8 9 2 0 0 0 0
9 10 1 0 0 0 0
10 5 1 0 0 0 0
7 11 2 0 0 0 0
9 12 1 0 0 0 0
12 13 2 0 0 0 0
13 14 1 0 0 0 0
14 15 2 0 0 0 0
15 16 1 0 0 0 0
16 17 2 0 0 0 0
17 12 1 0 0 0 0
3 18 1 0 0 0 0
1 19 1 0 0 0 0
6 20 1 0 0 0 0
16 21 1 0 0 0 0
22 23 1 1 0 0 0
23 24 1 1 0 0 0
25 24 1 1 0 0 0
25 26 1 0 0 0 0
26 27 1 0 0 0 0
27 22 1 0 0 0 0
22 28 1 0 0 0 0
23 29 1 0 0 0 0
24 30 1 0 0 0 0
32 33 1 1 0 0 0
33 34 1 1 0 0 0
35 34 1 1 0 0 0
35 36 1 0 0 0 0
36 37 1 0 0 0 0
37 32 1 0 0 0 0
33 38 1 0 0 0 0
34 39 1 0 0 0 0
31 32 1 0 0 0 0
35 30 1 0 0 0 0
25 19 1 0 0 0 0
15 40 1 0 0 0 0
40 41 1 0 0 0 0
27 42 1 0 0 0 0
42 43 1 0 0 0 0
37 44 1 0 0 0 0
44 45 1 0 0 0 0
M STY 1 1 SUP
M SLB 1 1 1
M SAL 1 2 40 41
M SBL 1 1 44
M SMT 1 OCH3
M SBV 1 44 -4.9815 7.4654
M STY 1 2 SUP
M SLB 1 2 2
M SAL 2 2 42 43
M SBL 2 1 46
M SMT 2 ^CH2OH
M SBV 2 46 -5.8699 7.8431
M STY 1 3 SUP
M SLB 1 3 3
M SAL 3 2 44 45
M SBL 3 1 48
M SMT 3 ^CH2OH
M SBV 3 48 -5.7567 7.4342
S SKP 8
ID FL3FFCGS0013
KNApSAcK_ID C00004431
NAME 8-Hydroxyluteolin 4'-methyl ether 7-allosyl-(1->2)-glucoside
CAS_RN 114611-04-0
FORMULA C28H32O17
EXACTMASS 640.163949598
AVERAGEMASS 640.54348
SMILES C(CO)(C1O)OC(Oc(c5)c(c(O3)c(c5O)C(C=C(c(c4)ccc(OC)c4O)3)=O)O)C(OC(C2O)OC(C(C2O)O)CO)C1O
M END