Category:TP
m (→Biosynthesis) |
(→Biosynthesis) |
||
| Line 53: | Line 53: | ||
==Biosynthesis== | ==Biosynthesis== | ||
| + | {{Twocolumn| | ||
| + | The common precursor of terpenes is isopentenyl diphosphate (IPP) and its isomer, dimethylallyl diphosphate (DMAPP). IPP is synthesized through mevalonate pathway (MVA pathway). | ||
| + | | | ||
| + | テルペンに共通する前駆体はイソペンテニル二リン酸 (IPP) とその異性体ジメチルアリル二リン酸 (DMAPP) です。IPP はメバロン酸経路で合成されます。 | ||
| + | }} | ||
* (h-t) ... head-to-tail conjugation | * (h-t) ... head-to-tail conjugation | ||
* (t-t) ... tail-to-tail conjugation | * (t-t) ... tail-to-tail conjugation | ||
Revision as of 13:38, 22 July 2010
Contents |
Terpenes
Overview
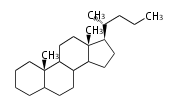
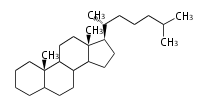
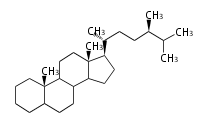
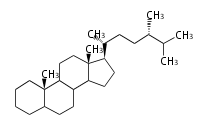
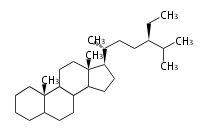
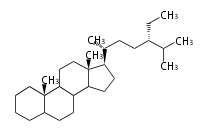
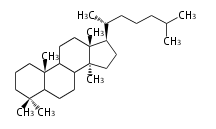
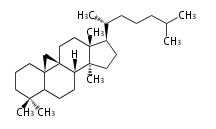
The word 'terpene' originates from turpentine, the distillation of resin from pine trees (Pinaceae). Chemically speaking, terpenes or terpenoids refer to all natural compounds build up from isoprene (C5) units. For this reason, terpenes are also called isoprenoids. <p>Well known terpenes include myrrh from balm trees, fragrance or essential oils of herbs and roses. Biologically, terpenes function as volatile expellant of herbivores and attractant of pollinators, or less volatile bitter antifeedants. Many hormones, pheromones and signal compounds are also derived from terpenes.
History
The German chemist Otto Wallach proposed the construction of terpenes from isoprene units known as the isoprene rule (1887), and received the Nobel Prize in Chemistry in 1910. In 1930s Leopold Ruzicka rationalized the reaction mechanisms of isoprene units and proposed the biogenetic isoprene rule, in which all compounds derived from active isoprene units should be included as terpenes. This active isoprene is now known as isopentenyl di- (or pyro-) phosphate (IPP). He received the Nobel Prize in Chemistry in 1939.
Classification
Depending on the number of isoprene units used, terpenoids are classified as follows.
- hemi-terpenes (C5)
- mono- (C10)
- sesqui- (C15)
- di- (C20)
- sester- (C25)
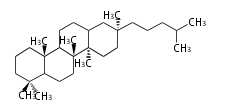
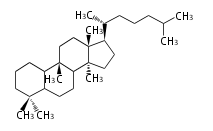
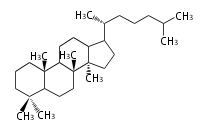
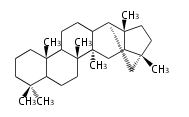
- tri- (C30)
- tetra- (C40)
- poly- (C5)n (n > 8)
Biosynthesis
The common precursor of terpenes is isopentenyl diphosphate (IPP) and its isomer, dimethylallyl diphosphate (DMAPP). IPP is synthesized through mevalonate pathway (MVA pathway).
- (h-t) ... head-to-tail conjugation
- (t-t) ... tail-to-tail conjugation
Design of Di-terpene ID numbers ID番号の設計
12-DIGIT
| T | P | 2 |
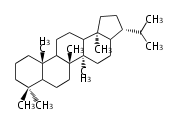
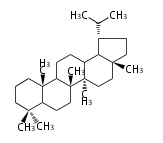
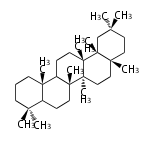
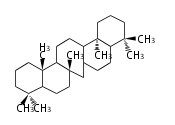
Design of Tri-terpene ID numbers ID番号の設計
12-DIGIT
| T | P | 3 | x | y | y | r | h | g | n | c | c |
- x ... species information
| Symbol at x | Kingdom | Phyla | Examples |
|---|---|---|---|
| I | Animalia | Arthropoda (Insects, crabs) | ecdysteroids |
| V | Chordate (Vertebrates) | sex steroids, corticosteroids, anabolic steroids | |
| O | Others | marine steroids | |
| P | Plantae | Phytosterols | lanosterols, cholesterols, brassinolides |
| S | Saponins | saponins | |
| F | Fungi | ergosterols | ergosterols |
| B | Bacteria | bacterial sterols | hopanoids |
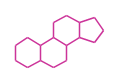
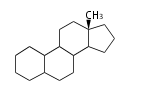
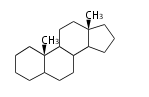
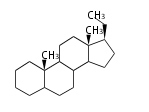
- y ... backbone structure (母核構造)
|
- r ... number of major rings (環構造数)
Click above categories to see details.
- h ... hydroxylation pattern (水酸基数)
Click above categories to see details.
- g ... glycosylation pattern(糖修飾パターン)
Click above categories to see details.
- n ... number of sugars (修飾糖数)
Click above categories to see details.
- c ... serial number (通し番号)
This category currently contains no pages or media.