Category:TP
From Metabolomics.JP
(Difference between revisions)
m |
|||
| Line 1: | Line 1: | ||
| − | = | + | =Terpenes= |
| − | + | ||
{{Twocolumn| | {{Twocolumn| | ||
| − | + | The word 'terpene' originates from turpentine, the distillation of resin from pine trees ([[:Category:Pinaceae|Pinaceae]]). Chemically speaking, terpenes refer to all natural compounds build up from isoprene (C5) units. | |
| + | |||
| + | Well known terpenes include myrrh from balm trees, fragrance or essential oils of herbs and roses. Biologically, terpenes function as volatile expellant of herbivores and attractant of pollinators, or less volatile bitter antifeedants. Many hormones, pheromones and signal compounds are also derived from terpenes. | ||
| | | | ||
| + | テルペンという言葉は松の木の脂を蒸留して得られるテレビン油 (turpentine)に由来しています。 | ||
| + | 化学的には、C5ユニットのイソプレンから構成される天然化合物を全てテルペンと呼びます。 | ||
| + | |||
| + | 著名な例には、香木から得られるミルラ(没薬)、ハーブやバラ等のエッセンシャルオイルや香り成分が含まれます。 | ||
| + | 生物学的な機能として、草食動物の忌避物質、花粉を運ぶ昆虫の誘引物質、苦味成分による摂食阻害物質として働きます。 | ||
| + | 多くのホルモン、フェロモン、シグナル伝達物質もテルペン由来です。 | ||
}} | }} | ||
| + | |||
| + | |||
==Design of Triterpene ID numbers ID番号の設計== | ==Design of Triterpene ID numbers ID番号の設計== | ||
Revision as of 15:35, 21 July 2010
Terpenes
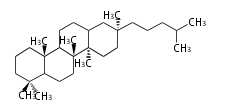
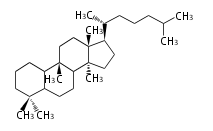
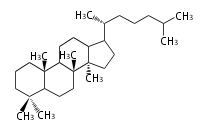
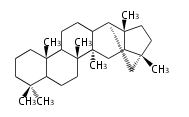
The word 'terpene' originates from turpentine, the distillation of resin from pine trees (Pinaceae). Chemically speaking, terpenes refer to all natural compounds build up from isoprene (C5) units. Well known terpenes include myrrh from balm trees, fragrance or essential oils of herbs and roses. Biologically, terpenes function as volatile expellant of herbivores and attractant of pollinators, or less volatile bitter antifeedants. Many hormones, pheromones and signal compounds are also derived from terpenes.
Design of Triterpene ID numbers ID番号の設計
12-DIGIT
| T | P | 3 | x | y | y | r | h | g | n | c | c |
- x ... species information
| Symbol at x | Kingdom | Phyla | Examples |
|---|---|---|---|
| I | Animalia | Arthropoda (Insects, crabs) | ecdysteroids |
| V | Chordate (Vertebrates) | sex steroids, corticosteroids, anabolic steroids | |
| O | Others | marine steroids | |
| P | Plantae | Phytosterols | lanosterols, cholesterols, brassinolides |
| S | Saponins | saponins | |
| F | Fungi | ergosterols | ergosterols |
| B | Bacteria | bacterial sterols | hopanoids |
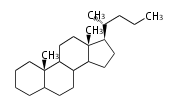
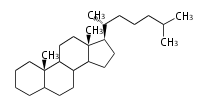
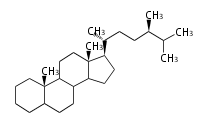
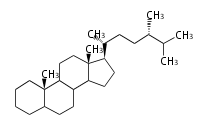
- y ... backbone structure (母核構造)
|
- r ... number of major rings (環構造数)
Click above categories to see details.
- h ... hydroxylation pattern (水酸基数)
Click above categories to see details.
- g ... glycosylation pattern(糖修飾パターン)
Click above categories to see details.
- n ... number of sugars (修飾糖数)
Click above categories to see details.
- c ... serial number (通し番号)
This category currently contains no pages or media.