Category:BMAA
| Line 99: | Line 99: | ||
===DL and RS do not coincide=== | ===DL and RS do not coincide=== | ||
{{Twocolumn| | {{Twocolumn| | ||
| − | In the RS notation, 19 L-amino acids become S-form (when H is placed behind the paper plane, the amino group, carboxyl group and side chain are arranged anticlockwise). Only L-cysteine becomes R-form because S in the side chain makes the arrangement in the order of the amino group, side chain, and carboxyl group. For this reason, although its tetrapodal configuration is the same as the rest of amino acids, it takes R-form. For the same reason, cystine is also R-form. | + | In the Cahn-Ingold-Prelog (or RS) notation, 19 L-amino acids become S-form (when H is placed behind the paper plane, the amino group, carboxyl group and side chain are arranged anticlockwise). Only L-cysteine becomes R-form because S in the side chain makes the arrangement in the order of the amino group, side chain, and carboxyl group. For this reason, although its tetrapodal configuration is the same as the rest of amino acids, it takes R-form. For the same reason, cystine is also R-form. |
| | | | ||
| − | + | アミノ酸をCahn-Ingold-Prelog則(RS)により表記してみると、19のL-アミノ酸がS体となります(Hを紙面の裏側に配置したとき、アミノ基、カルボキシル基、側鎖の順にまわると左回りになる)。ただひとつL-cysteineは、側鎖にSがくるためにアミノ基、側鎖、カルボキシル基の順番に回ります。そのため正四面体構造における配置は一緒なのですが、R体となります。システインのほかにシスチンも同様の理由でR体となります。 | |
}} | }} | ||
===Glycine has no chiral carbon=== | ===Glycine has no chiral carbon=== | ||
Revision as of 11:34, 21 May 2008
| Basic Metabolism Top (代謝トップ) |
Molecule Index (化合物索引) |
EC classes ( EC分類) |
Input New Data (新規入力) |
Upper classes : BM Basic Metabolites
Contents |
BMAA: Amino Acids
<p> Proteins are constituted from 20 amino acids. An amino acid has a carbon connected by an amino group and a carboxyl group (called an alpha carbon).
</p>| Straight Class | |||||
|---|---|---|---|---|---|
| BMAAS2 | Straight length 2 Gly 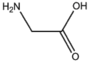
|
BMAAS3 | Straight length 3 Ser, Cys, Ala 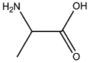
|
BMAAS4 | Straight length 4 Asp, Asn, Thr 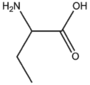
|
| BMAAS5 | Straight length 5 Gln, Glu 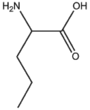
|
BMAAS6 | Straight length 6 Lys 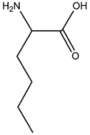
|
||
| Branched Class | |||||
| BMAAB4 | Branched length 4 Val, Met 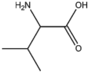
|
BMAAB5 | Branched length 5 Arg, Leu, Ile 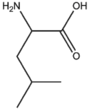
|
BMAAB6 | Branched length 6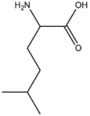
|
| BMAACC | Cyclic Phe, Tyr, Trp, His, Pro 120px |
||||
| Name | Code | Symbol | Structure | Character | pI | Required? |
|---|---|---|---|---|---|---|
| alanine | Ala | A | -CH3 | neutral | 6.0 | |
| serine | Ser | S | -CH2OH | neutral | 5.7 | |
| threonine | Thr | T | -CH(OH)CH3 | neutral | 5.6 | ○ |
| valine | Val | V | -CH(CH3)2 | neutral, branched | 6.0 | ○ |
| leucine | Leu | L | -CH2CH(CH3)2 | neutral, branched | 6.0 | ○ |
| isoleucine | Ile | I | -CH(CH3)CH2CH3 | neutral, branched | 6.0 | ○ |
| aspartic acid | Asp | D | -CH2COOH | acidic | 2.8 | |
| glutamic acid | Glu | E | -CH2CH2COOH | acidic | 3.2 | |
| asparagine | Asn | N | -CH2CONH2 | acidic, amido | 5.4 | |
| glutamine | Gln | Q | -CH2CH2CONH2 | acidic, amido | 5.7 | |
| lysine | Lys | K | -(CH2)4NH2 | basic | 9.7 | ○ |
| arginine | Arg | R | -(CH2)3NHC(=NH)NH2 | basic | 10.8 | |
| cysteine | Cys | C | -CH2SH | neutral, S | 5.1 | |
| methionine | Met | M | -(CH2)2SCH3 | neutral, S | 5.7 | ○ |
| phenylalanine | Phe | F | -CH2-Benz | neutral, aromatic | 5.5 | ○ |
| tyrosine | Tyr | Y | -CH2-Benz-OH | neutral, aromatic | 5.7 | |
| tryptophan | Trp | W | -CH2-Indole | neutral, aromatic | 5.9 | ○ |
| histidine | His | H | -CH2-Imidazole | basic, atomatic | 7.6 | ○ |
| proline | Pro | P | -NH-(CH2)3- | neutral, cyclic, 2nd amine | 6.2 |
D and L in Amino Acid
Definition
Except for Glycine, all amino acids contain chiral carbons, distinguished by D or L (R or S). In the Fischer projection, which places the carboxyl group upward and amino acid-specific branch downward, the amino group must comes either left or right, protruding from the paper plane. If the hydrogen is left and amino group is right, the amino acid is called D, after D-glyceraldehide. If the amino group is left, it is called L.
DL and RS do not coincide
In the Cahn-Ingold-Prelog (or RS) notation, 19 L-amino acids become S-form (when H is placed behind the paper plane, the amino group, carboxyl group and side chain are arranged anticlockwise). Only L-cysteine becomes R-form because S in the side chain makes the arrangement in the order of the amino group, side chain, and carboxyl group. For this reason, although its tetrapodal configuration is the same as the rest of amino acids, it takes R-form. For the same reason, cystine is also R-form.
Glycine has no chiral carbon
Glycine has two Hydrogen branches and therefore not chiral. The amino group of proline is included in the ring sturucture and therefore secondary amine.
This category currently contains no pages or media.