LCMS:Hochuekkito
From Metabolomics.JP
(Difference between revisions)
| Line 1: | Line 1: | ||
== 2D-Mass chromatogram == | == 2D-Mass chromatogram == | ||
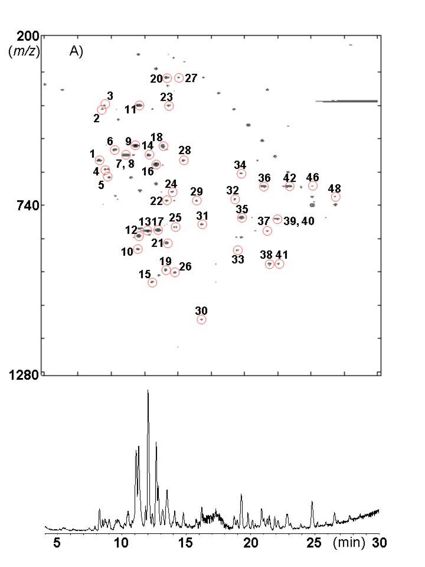
| − | [[Image:Hochuekkito_L_LC.jpg|thumb | + | [[Image:Hochuekkito_L_LC.jpg|thumb|left|425px|Mass chromatographic fingerprints of HET using Atractylodis Lanceae Rhizoma]] |
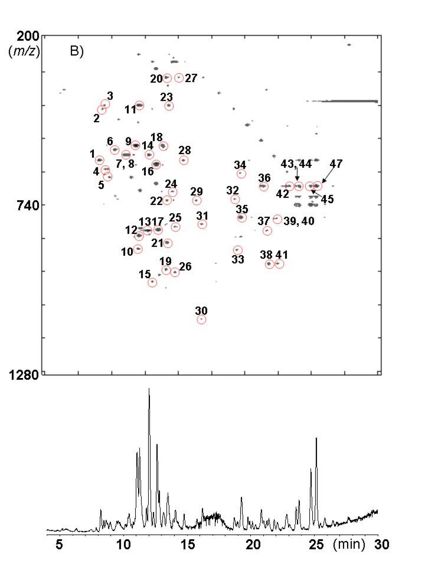
| − | + | [[Image:Hochuekkito_LC.jpg|thumb|425px|Mass chromatographic fingerprints of HET using Atractylodis Rhizoma]] | |
| − | [[Image:Hochuekkito_LC.jpg|thumb| | + | |
{| class="wikitable" style="width:80%" | {| class="wikitable" style="width:80%" | ||
| Line 26: | Line 25: | ||
{| class="wikitable" | {| class="wikitable" | ||
| − | ! Retention Time (min) !! Compounds !!Molecular formula !!(M-H)- ions | + | ! Retention Time (min) !! Compounds !!Origin!! Molecular formula !! (M-H)- ions |
| + | {{#repeat:Mass/Fragmentation|5|{{{Fragmentation|}}}|&&}} | ||
| + | |- | ||
| + | ! {{{1|8.33}}} | ||
| + | | [[{{{2|Trihydroxyflavone-diglucopyranoside}}}]] ||{{{3|Aurantii Nobilis}}} || {{{4|C27H29O15}}} || {{{5|593.1495}}} | ||
| + | |- | ||
| + | ! {{{1|8.59}}} | ||
| + | | [[{{{2|Trihydroxyflavone-diglucopyranoside}}}]] ||{{{3|Aurantii Nobilis}}} || {{{4|C27H29O15}}} || {{{5|593.1495}}} | ||
| + | |- | ||
| + | ! {{{1|8.75}}} | ||
| + | | [[{{{2|Trihydroxyflavone-diglucopyranoside}}}]] ||{{{3|Aurantii Nobilis}}} || {{{4|C27H29O15}}} || {{{5|593.1495}}} | ||
| + | |- | ||
| + | ! {{{1|8.97}}} | ||
| + | | [[{{{2|Trihydroxyflavone-diglucopyranoside}}}]] ||{{{3|Aurantii Nobilis}}} || {{{4|C27H29O15}}} || {{{5|593.1495}}} | ||
| + | |- | ||
| + | ! {{{1|9.19}}} | ||
| + | | [[{{{2|Trihydroxyflavone-diglucopyranoside}}}]] ||{{{3|Aurantii Nobilis}}} || {{{4|C27H29O15}}} || {{{5|593.1495}}} | ||
| + | |- | ||
| + | ! {{{1|9.49}}} | ||
| + | | [[{{{2|Trihydroxyflavone-diglucopyranoside}}}]] ||{{{3|Aurantii Nobilis}}} || {{{4|C27H29O15}}} || {{{5|593.1495}}} | ||
| + | |- | ||
| + | ! {{{1|10.20}}} | ||
| + | | [[{{{2|Trihydroxyflavone-diglucopyranoside}}}]] ||{{{3|Aurantii Nobilis}}} || {{{4|C27H29O15}}} || {{{5|593.1495}}} | ||
| + | |- | ||
| + | ! {{{1|10.43}}} | ||
| + | | [[{{{2|Trihydroxyflavone-diglucopyranoside}}}]] ||{{{3|Aurantii Nobilis}}} || {{{4|C27H29O15}}} || {{{5|593.1495}}} | ||
| + | |- | ||
| + | ! {{{1|11.08}}} | ||
| + | | [[{{{2|Trihydroxyflavone-diglucopyranoside}}}]] ||{{{3|Aurantii Nobilis}}} || {{{4|C27H29O15}}} || {{{5|593.1495}}} | ||
| + | |- | ||
| + | ! {{{1|11.31}}} | ||
| + | | [[{{{2|Trihydroxyflavone-diglucopyranoside}}}]] ||{{{3|Aurantii Nobilis}}} || {{{4|C27H29O15}}} || {{{5|593.1495}}} | ||
| + | |- | ||
| + | ! {{{1|11.51}}} | ||
| + | | [[{{{2|Trihydroxyflavone-diglucopyranoside}}}]] ||{{{3|Aurantii Nobilis}}} || {{{4|C27H29O15}}} || {{{5|593.1495}}} | ||
| + | |- | ||
| + | ! {{{1|11.65}}} | ||
| + | | [[{{{2|Trihydroxyflavone-diglucopyranoside}}}]] ||{{{3|Aurantii Nobilis}}} || {{{4|C27H29O15}}} || {{{5|593.1495}}} | ||
| + | |- | ||
| + | ! {{{1|12.02}}} | ||
| + | | [[{{{2|Trihydroxyflavone-diglucopyranoside}}}]] ||{{{3|Aurantii Nobilis}}} || {{{4|C27H29O15}}} || {{{5|593.1495}}} | ||
| + | |- | ||
| + | ! {{{1|12.36}}} | ||
| + | | [[{{{2|Trihydroxyflavone-diglucopyranoside}}}]] ||{{{3|Aurantii Nobilis}}} || {{{4|C27H29O15}}} || {{{5|593.1495}}} | ||
| + | |- | ||
| + | ! {{{1|12.57}}} | ||
| + | | [[{{{2|Trihydroxyflavone-diglucopyranoside}}}]] ||{{{3|Aurantii Nobilis}}} || {{{4|C27H29O15}}} || {{{5|593.1495}}} | ||
| + | |- | ||
| + | ! {{{1|12.60}}} | ||
| + | | [[{{{2|Trihydroxyflavone-diglucopyranoside}}}]] ||{{{3|Aurantii Nobilis}}} || {{{4|C27H29O15}}} || {{{5|593.1495}}} | ||
| + | |- | ||
| + | ! {{{1|13.12}}} | ||
| + | | [[{{{2|Trihydroxyflavone-diglucopyranoside}}}]] ||{{{3|Aurantii Nobilis}}} || {{{4|C27H29O15}}} || {{{5|593.1495}}} | ||
| + | |- | ||
| + | ! {{{1|13.18}}} | ||
| + | | [[{{{2|Trihydroxyflavone-diglucopyranoside}}}]] ||{{{3|Aurantii Nobilis}}} || {{{4|C27H29O15}}} || {{{5|593.1495}}} | ||
| + | |- | ||
| + | ! {{{1|13.31}}} | ||
| + | | [[{{{2|Trihydroxyflavone-diglucopyranoside}}}]] ||{{{3|Aurantii Nobilis}}} || {{{4|C27H29O15}}} || {{{5|593.1495}}} | ||
| + | |- | ||
| + | ! {{{1|13.40}}} | ||
| + | | [[{{{2|Trihydroxyflavone-diglucopyranoside}}}]] ||{{{3|Aurantii Nobilis}}} || {{{4|C27H29O15}}} || {{{5|593.1495}}} | ||
| + | |- | ||
| + | ! {{{1|13.65}}} | ||
| + | | [[{{{2|Trihydroxyflavone-diglucopyranoside}}}]] ||{{{3|Aurantii Nobilis}}} || {{{4|C27H29O15}}} || {{{5|593.1495}}} | ||
| + | |- | ||
| + | ! {{{1|13.64}}} | ||
| + | | [[{{{2|Trihydroxyflavone-diglucopyranoside}}}]] ||{{{3|Aurantii Nobilis}}} || {{{4|C27H29O15}}} || {{{5|593.1495}}} | ||
| + | |- | ||
| + | ! {{{1|13.76}}} | ||
| + | | [[{{{2|Trihydroxyflavone-diglucopyranoside}}}]] ||{{{3|Aurantii Nobilis}}} || {{{4|C27H29O15}}} || {{{5|593.1495}}} | ||
| + | |- | ||
| + | ! {{{1|13.99}}} | ||
| + | | [[{{{2|Trihydroxyflavone-diglucopyranoside}}}]] ||{{{3|Aurantii Nobilis}}} || {{{4|C27H29O15}}} || {{{5|593.1495}}} | ||
| + | |- | ||
| + | ! {{{1|14.23}}} | ||
| + | | [[{{{2|Trihydroxyflavone-diglucopyranoside}}}]] ||{{{3|Aurantii Nobilis}}} || {{{4|C27H29O15}}} || {{{5|593.1495}}} | ||
| + | |- | ||
| + | ! {{{1|14.48}}} | ||
| + | | [[{{{2|Trihydroxyflavone-diglucopyranoside}}}]] ||{{{3|Aurantii Nobilis}}} || {{{4|C27H29O15}}} || {{{5|593.1495}}} | ||
| + | |- | ||
| + | ! {{{1|14.71}}} | ||
| + | | [[{{{2|Trihydroxyflavone-diglucopyranoside}}}]] ||{{{3|Aurantii Nobilis}}} || {{{4|C27H29O15}}} || {{{5|593.1495}}} | ||
| + | |- | ||
| + | ! {{{1|14.95}}} | ||
| + | | [[{{{2|Trihydroxyflavone-diglucopyranoside}}}]] ||{{{3|Aurantii Nobilis}}} || {{{4|C27H29O15}}} || {{{5|593.1495}}} | ||
| + | |- | ||
| + | ! {{{1|15.70}}} | ||
| + | | [[{{{2|Trihydroxyflavone-diglucopyranoside}}}]] ||{{{3|Aurantii Nobilis}}} || {{{4|C27H29O15}}} || {{{5|593.1495}}} | ||
| + | |- | ||
| + | ! {{{1|16.06}}} | ||
| + | | [[{{{2|Trihydroxyflavone-diglucopyranoside}}}]] ||{{{3|Aurantii Nobilis}}} || {{{4|C27H29O15}}} || {{{5|593.1495}}} | ||
| + | |- | ||
| + | ! {{{1|16.13}}} | ||
| + | | [[{{{2|Trihydroxyflavone-diglucopyranoside}}}]] ||{{{3|Aurantii Nobilis}}} || {{{4|C27H29O15}}} || {{{5|593.1495}}} | ||
| + | |- | ||
| + | ! {{{1|17.87}}} | ||
| + | | [[{{{2|Trihydroxyflavone-diglucopyranoside}}}]] ||{{{3|Aurantii Nobilis}}} || {{{4|C27H29O15}}} || {{{5|593.1495}}} | ||
| + | |- | ||
| + | ! {{{1|18.88}}} | ||
| + | | [[{{{2|Trihydroxyflavone-diglucopyranoside}}}]] ||{{{3|Aurantii Nobilis}}} || {{{4|C27H29O15}}} || {{{5|593.1495}}} | ||
| + | |- | ||
| + | ! {{{1|19.11}}} | ||
| + | | [[{{{2|Trihydroxyflavone-diglucopyranoside}}}]] ||{{{3|Aurantii Nobilis}}} || {{{4|C27H29O15}}} || {{{5|593.1495}}} | ||
| + | |- | ||
| + | ! {{{1|19.19}}} | ||
| + | | [[{{{2|Trihydroxyflavone-diglucopyranoside}}}]] ||{{{3|Aurantii Nobilis}}} || {{{4|C27H29O15}}} || {{{5|593.1495}}} | ||
| + | |- | ||
| + | ! {{{1|20.73}}} | ||
| + | | [[{{{2|Trihydroxyflavone-diglucopyranoside}}}]] ||{{{3|Aurantii Nobilis}}} || {{{4|C27H29O15}}} || {{{5|593.1495}}} | ||
| + | |- | ||
| + | ! {{{1|21.39}}} | ||
| + | | [[{{{2|Trihydroxyflavone-diglucopyranoside}}}]] ||{{{3|Aurantii Nobilis}}} || {{{4|C27H29O15}}} || {{{5|593.1495}}} | ||
|- | |- | ||
| − | ! | + | ! {{{1|22.13}}} |
| − | | [[ | + | | [[{{{2|Trihydroxyflavone-diglucopyranoside}}}]] ||{{{3|Aurantii Nobilis}}} || {{{4|C27H29O15}}} || {{{5|593.1495}}} |
|- | |- | ||
| − | ! | + | ! {{{1|22.73}}} |
| − | | [[ | + | | [[{{{2|Trihydroxyflavone-diglucopyranoside}}}]] ||{{{3|Aurantii Nobilis}}} || {{{4|C27H29O15}}} || {{{5|593.1495}}} |
|- | |- | ||
| − | ! | + | ! {{{1|23.53}}} |
| − | | [[ | + | | [[{{{2|Trihydroxyflavone-diglucopyranoside}}}]] ||{{{3|Aurantii Nobilis}}} || {{{4|C27H29O15}}} || {{{5|593.1495}}} |
|- | |- | ||
| − | ! | + | ! {{{1|23.75}}} |
| − | | [[ | + | | [[{{{2|Trihydroxyflavone-diglucopyranoside}}}]] ||{{{3|Aurantii Nobilis}}} || {{{4|C27H29O15}}} || {{{5|593.1495}}} |
|- | |- | ||
| − | ! | + | ! {{{1|24.73}}} |
| − | | [[ | + | | [[{{{2|Trihydroxyflavone-diglucopyranoside}}}]] ||{{{3|Aurantii Nobilis}}} || {{{4|C27H29O15}}} || {{{5|593.1495}}} |
|- | |- | ||
| − | ! | + | ! {{{1|24.81}}} |
| − | | [[ | + | | [[{{{2|Trihydroxyflavone-diglucopyranoside}}}]] ||{{{3|Aurantii Nobilis}}} || {{{4|C27H29O15}}} || {{{5|593.1495}}} |
|- | |- | ||
| − | ! | + | ! {{{1|25.15}}} |
| − | | [[ | + | | [[{{{2|Trihydroxyflavone-diglucopyranoside}}}]] ||{{{3|Aurantii Nobilis}}} || {{{4|C27H29O15}}} || {{{5|593.1495}}} |
|- | |- | ||
| − | ! | + | ! {{{1|26.58}}} |
| − | | [[ | + | | [[{{{2|Trihydroxyflavone-diglucopyranoside}}}]] ||{{{3|Aurantii Nobilis}}} || {{{4|C27H29O15}}} || {{{5|593.1495}}} |
|} | |} | ||
Revision as of 14:20, 10 December 2009
2D-Mass chromatogram
| Instrument | Shimadzu LC-IT-TOF MS ESI (Negative ion mode) |
| Column | Waters Atlantis T3 (2.1 mm x 150 mm) |
| Column Temperature | 40℃ |
| Solvent A | 5 mM Ammonium acetate solution |
| Solvent B | acetonitrile |
| Gradient | 10% to 100% Solvent B (0-30 min), 100% Solvent B (30-40 min) |
| Source voltage | – 3.5 kV |
| Capillary temperature | 200 °C |
| Nebulizer gas | 1.5 l/min |
| Retention Time (min) | Compounds | Origin | Molecular formula | (M-H)- ions |
|---|---|---|---|---|
| 8.33 | Trihydroxyflavone-diglucopyranoside | Aurantii Nobilis | C27H29O15 | 593.1495 |
| 8.59 | Trihydroxyflavone-diglucopyranoside | Aurantii Nobilis | C27H29O15 | 593.1495 |
| 8.75 | Trihydroxyflavone-diglucopyranoside | Aurantii Nobilis | C27H29O15 | 593.1495 |
| 8.97 | Trihydroxyflavone-diglucopyranoside | Aurantii Nobilis | C27H29O15 | 593.1495 |
| 9.19 | Trihydroxyflavone-diglucopyranoside | Aurantii Nobilis | C27H29O15 | 593.1495 |
| 9.49 | Trihydroxyflavone-diglucopyranoside | Aurantii Nobilis | C27H29O15 | 593.1495 |
| 10.20 | Trihydroxyflavone-diglucopyranoside | Aurantii Nobilis | C27H29O15 | 593.1495 |
| 10.43 | Trihydroxyflavone-diglucopyranoside | Aurantii Nobilis | C27H29O15 | 593.1495 |
| 11.08 | Trihydroxyflavone-diglucopyranoside | Aurantii Nobilis | C27H29O15 | 593.1495 |
| 11.31 | Trihydroxyflavone-diglucopyranoside | Aurantii Nobilis | C27H29O15 | 593.1495 |
| 11.51 | Trihydroxyflavone-diglucopyranoside | Aurantii Nobilis | C27H29O15 | 593.1495 |
| 11.65 | Trihydroxyflavone-diglucopyranoside | Aurantii Nobilis | C27H29O15 | 593.1495 |
| 12.02 | Trihydroxyflavone-diglucopyranoside | Aurantii Nobilis | C27H29O15 | 593.1495 |
| 12.36 | Trihydroxyflavone-diglucopyranoside | Aurantii Nobilis | C27H29O15 | 593.1495 |
| 12.57 | Trihydroxyflavone-diglucopyranoside | Aurantii Nobilis | C27H29O15 | 593.1495 |
| 12.60 | Trihydroxyflavone-diglucopyranoside | Aurantii Nobilis | C27H29O15 | 593.1495 |
| 13.12 | Trihydroxyflavone-diglucopyranoside | Aurantii Nobilis | C27H29O15 | 593.1495 |
| 13.18 | Trihydroxyflavone-diglucopyranoside | Aurantii Nobilis | C27H29O15 | 593.1495 |
| 13.31 | Trihydroxyflavone-diglucopyranoside | Aurantii Nobilis | C27H29O15 | 593.1495 |
| 13.40 | Trihydroxyflavone-diglucopyranoside | Aurantii Nobilis | C27H29O15 | 593.1495 |
| 13.65 | Trihydroxyflavone-diglucopyranoside | Aurantii Nobilis | C27H29O15 | 593.1495 |
| 13.64 | Trihydroxyflavone-diglucopyranoside | Aurantii Nobilis | C27H29O15 | 593.1495 |
| 13.76 | Trihydroxyflavone-diglucopyranoside | Aurantii Nobilis | C27H29O15 | 593.1495 |
| 13.99 | Trihydroxyflavone-diglucopyranoside | Aurantii Nobilis | C27H29O15 | 593.1495 |
| 14.23 | Trihydroxyflavone-diglucopyranoside | Aurantii Nobilis | C27H29O15 | 593.1495 |
| 14.48 | Trihydroxyflavone-diglucopyranoside | Aurantii Nobilis | C27H29O15 | 593.1495 |
| 14.71 | Trihydroxyflavone-diglucopyranoside | Aurantii Nobilis | C27H29O15 | 593.1495 |
| 14.95 | Trihydroxyflavone-diglucopyranoside | Aurantii Nobilis | C27H29O15 | 593.1495 |
| 15.70 | Trihydroxyflavone-diglucopyranoside | Aurantii Nobilis | C27H29O15 | 593.1495 |
| 16.06 | Trihydroxyflavone-diglucopyranoside | Aurantii Nobilis | C27H29O15 | 593.1495 |
| 16.13 | Trihydroxyflavone-diglucopyranoside | Aurantii Nobilis | C27H29O15 | 593.1495 |
| 17.87 | Trihydroxyflavone-diglucopyranoside | Aurantii Nobilis | C27H29O15 | 593.1495 |
| 18.88 | Trihydroxyflavone-diglucopyranoside | Aurantii Nobilis | C27H29O15 | 593.1495 |
| 19.11 | Trihydroxyflavone-diglucopyranoside | Aurantii Nobilis | C27H29O15 | 593.1495 |
| 19.19 | Trihydroxyflavone-diglucopyranoside | Aurantii Nobilis | C27H29O15 | 593.1495 |
| 20.73 | Trihydroxyflavone-diglucopyranoside | Aurantii Nobilis | C27H29O15 | 593.1495 |
| 21.39 | Trihydroxyflavone-diglucopyranoside | Aurantii Nobilis | C27H29O15 | 593.1495 |
| 22.13 | Trihydroxyflavone-diglucopyranoside | Aurantii Nobilis | C27H29O15 | 593.1495 |
| 22.73 | Trihydroxyflavone-diglucopyranoside | Aurantii Nobilis | C27H29O15 | 593.1495 |
| 23.53 | Trihydroxyflavone-diglucopyranoside | Aurantii Nobilis | C27H29O15 | 593.1495 |
| 23.75 | Trihydroxyflavone-diglucopyranoside | Aurantii Nobilis | C27H29O15 | 593.1495 |
| 24.73 | Trihydroxyflavone-diglucopyranoside | Aurantii Nobilis | C27H29O15 | 593.1495 |
| 24.81 | Trihydroxyflavone-diglucopyranoside | Aurantii Nobilis | C27H29O15 | 593.1495 |
| 25.15 | Trihydroxyflavone-diglucopyranoside | Aurantii Nobilis | C27H29O15 | 593.1495 |
| 26.58 | Trihydroxyflavone-diglucopyranoside | Aurantii Nobilis | C27H29O15 | 593.1495 |