Mol:FL3FACGS0084
From Metabolomics.JP
(Difference between revisions)
| Line 1: | Line 1: | ||
| − | + | ||
| − | + | ||
| − | Copyright: ARM project http://www.metabolome.jp/ | + | Copyright: ARM project http://www.metabolome.jp/ |
| − | 57 62 0 0 0 0 0 0 0 0999 V2000 | + | 57 62 0 0 0 0 0 0 0 0999 V2000 |
| − | 5.1047 1.5874 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 5.1047 1.5874 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 5.1047 0.7624 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 5.1047 0.7624 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 5.8191 0.3499 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 5.8191 0.3499 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 6.5336 0.7624 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 6.5336 0.7624 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 6.5336 1.5874 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 6.5336 1.5874 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 5.8191 1.9999 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 5.8191 1.9999 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 4.3902 0.3499 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 4.3902 0.3499 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 3.6757 0.7624 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | 3.6757 0.7624 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 2.9613 0.3499 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 2.9613 0.3499 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 2.9613 -0.4751 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 2.9613 -0.4751 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 3.6757 -0.8876 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 3.6757 -0.8876 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 4.3902 -0.4751 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 4.3902 -0.4751 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 2.2468 0.7624 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 2.2468 0.7624 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 1.5323 0.3499 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 1.5323 0.3499 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 1.5323 -0.4751 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 1.5323 -0.4751 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 2.2468 -0.8876 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 2.2468 -0.8876 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 3.6757 -1.6059 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | 3.6757 -1.6059 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 0.8179 0.7624 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | 0.8179 0.7624 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 2.2468 -1.5736 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | 2.2468 -1.5736 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 7.2392 1.9889 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | 7.2392 1.9889 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 7.1081 0.4307 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | 7.1081 0.4307 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -1.8000 0.8838 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -1.8000 0.8838 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -1.3875 0.1694 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -1.3875 0.1694 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -0.5894 0.3785 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -0.5894 0.3785 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 0.2038 0.1517 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 0.2038 0.1517 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -0.2087 0.8662 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | -0.2087 0.8662 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -1.0068 0.6572 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -1.0068 0.6572 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -1.1637 1.2428 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -1.1637 1.2428 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -1.6434 1.5198 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | -1.6434 1.5198 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -2.2831 0.4008 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | -2.2831 0.4008 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -1.8361 0.4283 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | -1.8361 0.4283 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -0.7609 -0.2613 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | -0.7609 -0.2613 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -3.3675 -0.3867 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -3.3675 -0.3867 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -2.9550 -1.1011 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -2.9550 -1.1011 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -2.1569 -0.8920 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -2.1569 -0.8920 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -1.3637 -1.1188 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -1.3637 -1.1188 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -1.7762 -0.4043 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | -1.7762 -0.4043 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -2.5743 -0.6133 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -2.5743 -0.6133 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -2.7312 -0.0277 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -2.7312 -0.0277 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -3.2109 0.2493 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | -3.2109 0.2493 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -3.8506 -0.8697 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | -3.8506 -0.8697 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -3.4036 -0.8422 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | -3.4036 -0.8422 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -2.3284 -1.5318 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | -2.3284 -1.5318 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -6.1478 -0.8331 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -6.1478 -0.8331 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -5.7353 -1.5476 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -5.7353 -1.5476 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -4.9372 -1.3385 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -4.9372 -1.3385 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -4.1440 -1.5653 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -4.1440 -1.5653 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -4.5564 -0.8507 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | -4.5564 -0.8507 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -5.3546 -1.0597 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -5.3546 -1.0597 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -5.5405 -0.3658 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -5.5405 -0.3658 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -6.0832 -0.0525 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | -6.0832 -0.0525 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -6.5655 -1.2508 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | -6.5655 -1.2508 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -5.9964 -1.9999 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | -5.9964 -1.9999 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -4.7839 -1.9106 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | -4.7839 -1.9106 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -6.6603 0.2807 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -6.6603 0.2807 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -6.6603 0.9562 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | -6.6603 0.9562 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -7.2392 -0.0535 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -7.2392 -0.0535 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 1 2 2 0 0 0 0 | + | 1 2 2 0 0 0 0 |
| − | 2 3 1 0 0 0 0 | + | 2 3 1 0 0 0 0 |
| − | 3 4 2 0 0 0 0 | + | 3 4 2 0 0 0 0 |
| − | 4 5 1 0 0 0 0 | + | 4 5 1 0 0 0 0 |
| − | 5 6 2 0 0 0 0 | + | 5 6 2 0 0 0 0 |
| − | 6 1 1 0 0 0 0 | + | 6 1 1 0 0 0 0 |
| − | 2 7 1 0 0 0 0 | + | 2 7 1 0 0 0 0 |
| − | 7 8 1 0 0 0 0 | + | 7 8 1 0 0 0 0 |
| − | 8 9 1 0 0 0 0 | + | 8 9 1 0 0 0 0 |
| − | 9 10 2 0 0 0 0 | + | 9 10 2 0 0 0 0 |
| − | 10 11 1 0 0 0 0 | + | 10 11 1 0 0 0 0 |
| − | 11 12 1 0 0 0 0 | + | 11 12 1 0 0 0 0 |
| − | 12 7 2 0 0 0 0 | + | 12 7 2 0 0 0 0 |
| − | 9 13 1 0 0 0 0 | + | 9 13 1 0 0 0 0 |
| − | 13 14 2 0 0 0 0 | + | 13 14 2 0 0 0 0 |
| − | 14 15 1 0 0 0 0 | + | 14 15 1 0 0 0 0 |
| − | 15 16 2 0 0 0 0 | + | 15 16 2 0 0 0 0 |
| − | 16 10 1 0 0 0 0 | + | 16 10 1 0 0 0 0 |
| − | 14 18 1 0 0 0 0 | + | 14 18 1 0 0 0 0 |
| − | 11 17 2 0 0 0 0 | + | 11 17 2 0 0 0 0 |
| − | 16 19 1 0 0 0 0 | + | 16 19 1 0 0 0 0 |
| − | 5 20 1 0 0 0 0 | + | 5 20 1 0 0 0 0 |
| − | 4 21 1 0 0 0 0 | + | 4 21 1 0 0 0 0 |
| − | 22 23 1 1 0 0 0 | + | 22 23 1 1 0 0 0 |
| − | 23 24 1 1 0 0 0 | + | 23 24 1 1 0 0 0 |
| − | 25 24 1 1 0 0 0 | + | 25 24 1 1 0 0 0 |
| − | 25 26 1 0 0 0 0 | + | 25 26 1 0 0 0 0 |
| − | 26 27 1 0 0 0 0 | + | 26 27 1 0 0 0 0 |
| − | 27 22 1 0 0 0 0 | + | 27 22 1 0 0 0 0 |
| − | 27 28 1 0 0 0 0 | + | 27 28 1 0 0 0 0 |
| − | 28 29 1 0 0 0 0 | + | 28 29 1 0 0 0 0 |
| − | 22 30 1 0 0 0 0 | + | 22 30 1 0 0 0 0 |
| − | 23 31 1 0 0 0 0 | + | 23 31 1 0 0 0 0 |
| − | 24 32 1 0 0 0 0 | + | 24 32 1 0 0 0 0 |
| − | 25 18 1 0 0 0 0 | + | 25 18 1 0 0 0 0 |
| − | 33 34 1 1 0 0 0 | + | 33 34 1 1 0 0 0 |
| − | 34 35 1 1 0 0 0 | + | 34 35 1 1 0 0 0 |
| − | 36 35 1 1 0 0 0 | + | 36 35 1 1 0 0 0 |
| − | 36 37 1 0 0 0 0 | + | 36 37 1 0 0 0 0 |
| − | 37 38 1 0 0 0 0 | + | 37 38 1 0 0 0 0 |
| − | 38 33 1 0 0 0 0 | + | 38 33 1 0 0 0 0 |
| − | 38 39 1 0 0 0 0 | + | 38 39 1 0 0 0 0 |
| − | 39 40 1 0 0 0 0 | + | 39 40 1 0 0 0 0 |
| − | 33 41 1 0 0 0 0 | + | 33 41 1 0 0 0 0 |
| − | 34 42 1 0 0 0 0 | + | 34 42 1 0 0 0 0 |
| − | 35 43 1 0 0 0 0 | + | 35 43 1 0 0 0 0 |
| − | 36 32 1 0 0 0 0 | + | 36 32 1 0 0 0 0 |
| − | 44 45 1 1 0 0 0 | + | 44 45 1 1 0 0 0 |
| − | 45 46 1 1 0 0 0 | + | 45 46 1 1 0 0 0 |
| − | 47 46 1 1 0 0 0 | + | 47 46 1 1 0 0 0 |
| − | 47 48 1 0 0 0 0 | + | 47 48 1 0 0 0 0 |
| − | 48 49 1 0 0 0 0 | + | 48 49 1 0 0 0 0 |
| − | 49 44 1 0 0 0 0 | + | 49 44 1 0 0 0 0 |
| − | 49 50 1 0 0 0 0 | + | 49 50 1 0 0 0 0 |
| − | 50 51 1 0 0 0 0 | + | 50 51 1 0 0 0 0 |
| − | 44 52 1 0 0 0 0 | + | 44 52 1 0 0 0 0 |
| − | 45 53 1 0 0 0 0 | + | 45 53 1 0 0 0 0 |
| − | 46 54 1 0 0 0 0 | + | 46 54 1 0 0 0 0 |
| − | 51 55 1 0 0 0 0 | + | 51 55 1 0 0 0 0 |
| − | 55 56 2 0 0 0 0 | + | 55 56 2 0 0 0 0 |
| − | 55 57 1 0 0 0 0 | + | 55 57 1 0 0 0 0 |
| − | 47 42 1 0 0 0 0 | + | 47 42 1 0 0 0 0 |
| − | S SKP 8 | + | S SKP 8 |
| − | ID FL3FACGS0084 | + | ID FL3FACGS0084 |
| − | KNApSAcK_ID C00013669 | + | KNApSAcK_ID C00013669 |
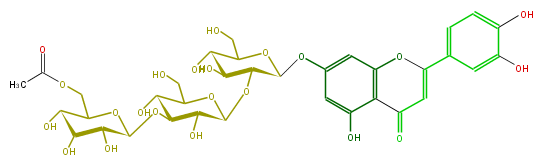
| − | NAME Luteorin 7-(6''''-acetylallosyl-(1->3)-glucosyl-(1->2)-glucoside;Veronicoside A;7-[(O-6-O-Acetyl-beta-D-allopyranosyl-(1->3)-O-beta-D-glucopyranosyl-(1->2)-beta-D-glucopyranosyl)oxy]-2-(3,4-dihydroxyphenyl)-5-hydroxy-4H-1-benzopyran-4-one | + | NAME Luteorin 7-(6''''-acetylallosyl-(1->3)-glucosyl-(1->2)-glucoside;Veronicoside A;7-[(O-6-O-Acetyl-beta-D-allopyranosyl-(1->3)-O-beta-D-glucopyranosyl-(1->2)-beta-D-glucopyranosyl)oxy]-2-(3,4-dihydroxyphenyl)-5-hydroxy-4H-1-benzopyran-4-one |
| − | CAS_RN 173792-54-6 | + | CAS_RN 173792-54-6 |
| − | FORMULA C35H42O22 | + | FORMULA C35H42O22 |
| − | EXACTMASS 814.216773028 | + | EXACTMASS 814.216773028 |
| − | AVERAGEMASS 814.69478 | + | AVERAGEMASS 814.69478 |
| − | SMILES c(c64)c(cc(c(C(=O)C=C(O6)c(c5)ccc(O)c(O)5)4)O)OC(O1)C(OC(C(O)2)OC(C(C(OC(C3O)OC(C(C3O)O)COC(C)=O)2)O)CO)C(C(C1CO)O)O | + | SMILES c(c64)c(cc(c(C(=O)C=C(O6)c(c5)ccc(O)c(O)5)4)O)OC(O1)C(OC(C(O)2)OC(C(C(OC(C3O)OC(C(C3O)O)COC(C)=O)2)O)CO)C(C(C1CO)O)O |
M END | M END | ||
| − | |||
Latest revision as of 09:00, 14 March 2009
Copyright: ARM project http://www.metabolome.jp/
57 62 0 0 0 0 0 0 0 0999 V2000
5.1047 1.5874 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
5.1047 0.7624 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
5.8191 0.3499 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
6.5336 0.7624 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
6.5336 1.5874 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
5.8191 1.9999 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
4.3902 0.3499 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
3.6757 0.7624 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
2.9613 0.3499 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
2.9613 -0.4751 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
3.6757 -0.8876 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
4.3902 -0.4751 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
2.2468 0.7624 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
1.5323 0.3499 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
1.5323 -0.4751 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
2.2468 -0.8876 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
3.6757 -1.6059 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
0.8179 0.7624 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
2.2468 -1.5736 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
7.2392 1.9889 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
7.1081 0.4307 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
-1.8000 0.8838 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-1.3875 0.1694 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-0.5894 0.3785 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
0.2038 0.1517 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-0.2087 0.8662 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
-1.0068 0.6572 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-1.1637 1.2428 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-1.6434 1.5198 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
-2.2831 0.4008 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
-1.8361 0.4283 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
-0.7609 -0.2613 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
-3.3675 -0.3867 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-2.9550 -1.1011 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-2.1569 -0.8920 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-1.3637 -1.1188 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-1.7762 -0.4043 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
-2.5743 -0.6133 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-2.7312 -0.0277 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-3.2109 0.2493 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
-3.8506 -0.8697 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
-3.4036 -0.8422 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
-2.3284 -1.5318 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
-6.1478 -0.8331 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-5.7353 -1.5476 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-4.9372 -1.3385 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-4.1440 -1.5653 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-4.5564 -0.8507 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
-5.3546 -1.0597 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-5.5405 -0.3658 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-6.0832 -0.0525 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
-6.5655 -1.2508 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
-5.9964 -1.9999 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
-4.7839 -1.9106 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
-6.6603 0.2807 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-6.6603 0.9562 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
-7.2392 -0.0535 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
1 2 2 0 0 0 0
2 3 1 0 0 0 0
3 4 2 0 0 0 0
4 5 1 0 0 0 0
5 6 2 0 0 0 0
6 1 1 0 0 0 0
2 7 1 0 0 0 0
7 8 1 0 0 0 0
8 9 1 0 0 0 0
9 10 2 0 0 0 0
10 11 1 0 0 0 0
11 12 1 0 0 0 0
12 7 2 0 0 0 0
9 13 1 0 0 0 0
13 14 2 0 0 0 0
14 15 1 0 0 0 0
15 16 2 0 0 0 0
16 10 1 0 0 0 0
14 18 1 0 0 0 0
11 17 2 0 0 0 0
16 19 1 0 0 0 0
5 20 1 0 0 0 0
4 21 1 0 0 0 0
22 23 1 1 0 0 0
23 24 1 1 0 0 0
25 24 1 1 0 0 0
25 26 1 0 0 0 0
26 27 1 0 0 0 0
27 22 1 0 0 0 0
27 28 1 0 0 0 0
28 29 1 0 0 0 0
22 30 1 0 0 0 0
23 31 1 0 0 0 0
24 32 1 0 0 0 0
25 18 1 0 0 0 0
33 34 1 1 0 0 0
34 35 1 1 0 0 0
36 35 1 1 0 0 0
36 37 1 0 0 0 0
37 38 1 0 0 0 0
38 33 1 0 0 0 0
38 39 1 0 0 0 0
39 40 1 0 0 0 0
33 41 1 0 0 0 0
34 42 1 0 0 0 0
35 43 1 0 0 0 0
36 32 1 0 0 0 0
44 45 1 1 0 0 0
45 46 1 1 0 0 0
47 46 1 1 0 0 0
47 48 1 0 0 0 0
48 49 1 0 0 0 0
49 44 1 0 0 0 0
49 50 1 0 0 0 0
50 51 1 0 0 0 0
44 52 1 0 0 0 0
45 53 1 0 0 0 0
46 54 1 0 0 0 0
51 55 1 0 0 0 0
55 56 2 0 0 0 0
55 57 1 0 0 0 0
47 42 1 0 0 0 0
S SKP 8
ID FL3FACGS0084
KNApSAcK_ID C00013669
NAME Luteorin 7-(6''''-acetylallosyl-(1->3)-glucosyl-(1->2)-glucoside;Veronicoside A;7-[(O-6-O-Acetyl-beta-D-allopyranosyl-(1->3)-O-beta-D-glucopyranosyl-(1->2)-beta-D-glucopyranosyl)oxy]-2-(3,4-dihydroxyphenyl)-5-hydroxy-4H-1-benzopyran-4-one
CAS_RN 173792-54-6
FORMULA C35H42O22
EXACTMASS 814.216773028
AVERAGEMASS 814.69478
SMILES c(c64)c(cc(c(C(=O)C=C(O6)c(c5)ccc(O)c(O)5)4)O)OC(O1)C(OC(C(O)2)OC(C(C(OC(C3O)OC(C(C3O)O)COC(C)=O)2)O)CO)C(C(C1CO)O)O
M END