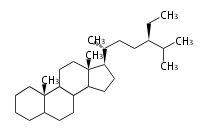
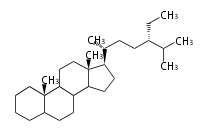
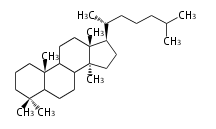
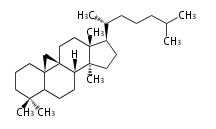
Category:TP
m |
|||
| Line 1: | Line 1: | ||
=Terpenes= | =Terpenes= | ||
| + | ==Overview== | ||
{{Twocolumn| | {{Twocolumn| | ||
| − | The word 'terpene' originates from turpentine, the distillation of resin from pine trees ([[:Category:Pinaceae|Pinaceae]]). Chemically speaking, terpenes refer to all natural compounds build up from isoprene (C5) units. | + | The word 'terpene' originates from turpentine, the distillation of resin from pine trees ([[:Category:Pinaceae|Pinaceae]]). Chemically speaking, terpenes or terpenoids refer to all natural compounds build up from isoprene (C5) units. |
| + | For this reason, terpenes are also called isoprenoids. | ||
| − | Well known terpenes include myrrh from balm trees, fragrance or essential oils of herbs and roses. Biologically, terpenes function as volatile expellant of herbivores and attractant of pollinators, or less volatile bitter antifeedants. Many hormones, pheromones and signal compounds are also derived from terpenes. | + | <p>Well known terpenes include myrrh from balm trees, fragrance or essential oils of herbs and roses. Biologically, terpenes function as volatile expellant of herbivores and attractant of pollinators, or less volatile bitter antifeedants. Many hormones, pheromones and signal compounds are also derived from terpenes. |
| | | | ||
テルペンという言葉は松の木の脂を蒸留して得られるテレビン油 (turpentine)に由来しています。 | テルペンという言葉は松の木の脂を蒸留して得られるテレビン油 (turpentine)に由来しています。 | ||
| − | + | 化学的には、C5ユニットのイソプレンから構成される天然化合物を全てテルペンまたはテルペノイドと呼びます。 | |
| + | この理由で、テルペンのことをイソプレノイドとも呼びます。 | ||
| − | + | <p>身近な具体例として、香木から得られるミルラ(没薬)、ハーブやバラ等のエッセンシャルオイルや香り成分が含まれます。 | |
| − | + | 生物学的な機能としては、草食動物の忌避物質、花粉を運ぶ昆虫の誘引物質、苦味成分による摂食阻害物質として働きます。 | |
多くのホルモン、フェロモン、シグナル伝達物質もテルペン由来です。 | 多くのホルモン、フェロモン、シグナル伝達物質もテルペン由来です。 | ||
}} | }} | ||
| + | ===History=== | ||
| + | {{Twocolumn| | ||
| + | The German chemist Otto Wallach proposed the construction of terpenes from isoprene units known as the ''isoprene rule'' (1887), and received [http://nobelprize.org/nobel_prizes/chemistry/laureates/1910/wallach-lecture.html the Nobel Prize in Chemistry in 1910]. | ||
| + | In 1930s Leopold Ruzicka rationalized the reaction mechanisms of isoprene units and proposed the ''biogenetic isoprene rule'', in which all compounds derived from ''active'' isoprene units should be included as terpenes. He received [http://nobelprize.org/nobel_prizes/chemistry/laureates/1939/ruzicka-lecture.html the Nobel Prize in Chemistry in 1939]. | ||
| + | | | ||
| + | ドイツの化学者オットー・ヴァラッハは、テルペンがイソプレン単位から構成されること(イソプレン則)を示し、[http://nobelprize.org/nobel_prizes/chemistry/laureates/1910/wallach-lecture.html 1910年ノーベル化学賞]を受賞した。 | ||
| + | 1930年代にスイスのレオポルド・ルジツカがイソプレン単位の反応機構を整理し、反応性の高いイソプレンの生体内反応による産物を全てテルペンとするイソプレン生合成則を示した。 | ||
| + | ルジツカはこの業績により[http://nobelprize.org/nobel_prizes/chemistry/laureates/1939/ruzicka-lecture.html 1937年ノーベル化学賞]を受賞した。<ref>[http://www.springerlink.com/content/nrmg1jpq1454663p/ Ruzicka, L "The isoprene rule and the biogenesis of terpenic compounds" ''Cell. Mol. Life Sci.'' 9(10): 357-367, 1953]</ref> | ||
| + | }} | ||
| + | |||
| + | <references/> | ||
| + | |||
| + | ===Classification=== | ||
| + | {{Twocolumn| | ||
| + | Depending on the number of isoprene units used, terpenoids are classified as follows. | ||
| + | * hemi-terpenes (C5) | ||
| + | * mono- (C10) | ||
| + | * sesqui- (C15) | ||
| + | * di- (C20) | ||
| + | * sester- (C25) | ||
| + | * tri- (C30) | ||
| + | * tetra- (C40) | ||
| + | * poly- (C5)<sub>n</sub> (n > 8) | ||
| + | | | ||
| + | 利用するイソプレン単位の数に応じて、以下のように分類されています。 | ||
| + | * ヘミ hemi-terpenes (C5) | ||
| + | * モノ mono- (C10) | ||
| + | * セスキ sesqui- (C15) | ||
| + | * ジ di- (C20) | ||
| + | * セスタ sester- (C25) | ||
| + | * トリ tri- (C30) | ||
| + | * テトラ tetra- (C40) | ||
| + | * ポリ poly- (C5)<sub>n</sub> (n > 8) | ||
| + | }} | ||
| + | |||
| + | ===Biosynthesis=== | ||
| + | * (h-t) ... head-to-tail conjugation | ||
| + | * (t-t) ... tail-to-tail conjugation | ||
| + | {| | ||
| + | |- | ||
| + | | hemiterpenes | ||
| + | | | ||
| + | | C5 | ||
| + | | [[Image:Arrow00r.png]]<br/> + other materials | ||
| + | | e.g. ester alkaloids | ||
| + | |- | ||
| + | | || || [[Image:Arrow00d35.png]] +C5 (h-t) | ||
| + | |- | ||
| + | |- | ||
| + | | monoterpenes | ||
| + | | | ||
| + | | C10 | ||
| + | | [[Image:Arrow00r.png]]<br/> + other materials | ||
| + | | e.g. indole alkaloids, cannabinoids | ||
| + | |- | ||
| + | | || || [[Image:Arrow00d35.png]] +C5 (h-t) | ||
| + | |- | ||
| + | | sesquiterpenes | ||
| + | | | ||
| + | | C15 | ||
| + | | [[Image:Arrow00r.png]]<br/>x2 (t-t) | ||
| + | | C30 | ||
| + | | triterpenes | ||
| + | | [[Image:Arrow00r35.png]] | ||
| + | | steroids | ||
| + | |- | ||
| + | | || || [[Image:Arrow00d35.png]] +C5 (h-t) | ||
| + | |- | ||
| + | | diterpenes | ||
| + | | | ||
| + | | C20 | ||
| + | | [[Image:Arrow00r.png]]<br/>x2 (t-t) | ||
| + | | C40 | ||
| + | | tetraterpenes | ||
| + | | [[Image:Arrow00r35.png]] | ||
| + | | carotenoids | ||
| + | |- | ||
| + | | || || [[Image:Arrow00d35.png]] +C5 h-t | ||
| + | |- | ||
| + | | sesterterpenes | ||
| + | | | ||
| + | | C25 | ||
| + | |} | ||
| + | ==Design of Di-terpene ID numbers ID番号の設計== | ||
| + | <center> | ||
| + | 12-DIGIT | ||
| + | <table border=1> | ||
| + | <tr> | ||
| + | <td style="background-color:green;" width=15px align="center"><big>T</big> | ||
| + | <td style="background-color:green;" width=15px align="center"><big>P</big> | ||
| + | <td style="background-color:green;" width=15px align="center"><big>2</big> | ||
| + | </tr> | ||
| + | </table> | ||
| + | </center> | ||
| − | ==Design of | + | ==Design of Tri-terpene ID numbers ID番号の設計== |
<center> | <center> | ||
12-DIGIT | 12-DIGIT | ||
Revision as of 09:03, 22 July 2010
Contents |
Terpenes
Overview
The word 'terpene' originates from turpentine, the distillation of resin from pine trees (Pinaceae). Chemically speaking, terpenes or terpenoids refer to all natural compounds build up from isoprene (C5) units. For this reason, terpenes are also called isoprenoids. <p>Well known terpenes include myrrh from balm trees, fragrance or essential oils of herbs and roses. Biologically, terpenes function as volatile expellant of herbivores and attractant of pollinators, or less volatile bitter antifeedants. Many hormones, pheromones and signal compounds are also derived from terpenes.
History
The German chemist Otto Wallach proposed the construction of terpenes from isoprene units known as the isoprene rule (1887), and received the Nobel Prize in Chemistry in 1910. In 1930s Leopold Ruzicka rationalized the reaction mechanisms of isoprene units and proposed the biogenetic isoprene rule, in which all compounds derived from active isoprene units should be included as terpenes. He received the Nobel Prize in Chemistry in 1939.
Classification
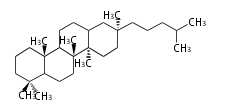
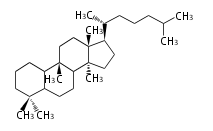
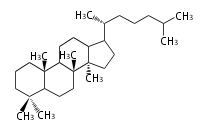
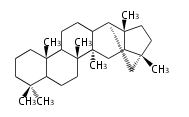
Depending on the number of isoprene units used, terpenoids are classified as follows.
- hemi-terpenes (C5)
- mono- (C10)
- sesqui- (C15)
- di- (C20)
- sester- (C25)
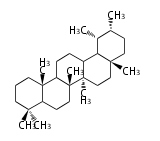
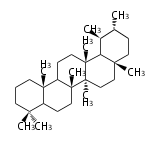
- tri- (C30)
- tetra- (C40)
- poly- (C5)n (n > 8)
Biosynthesis
- (h-t) ... head-to-tail conjugation
- (t-t) ... tail-to-tail conjugation
Design of Di-terpene ID numbers ID番号の設計
12-DIGIT
| T | P | 2 |
Design of Tri-terpene ID numbers ID番号の設計
12-DIGIT
| T | P | 3 | x | y | y | r | h | g | n | c | c |
- x ... species information
| Symbol at x | Kingdom | Phyla | Examples |
|---|---|---|---|
| I | Animalia | Arthropoda (Insects, crabs) | ecdysteroids |
| V | Chordate (Vertebrates) | sex steroids, corticosteroids, anabolic steroids | |
| O | Others | marine steroids | |
| P | Plantae | Phytosterols | lanosterols, cholesterols, brassinolides |
| S | Saponins | saponins | |
| F | Fungi | ergosterols | ergosterols |
| B | Bacteria | bacterial sterols | hopanoids |
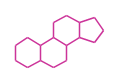
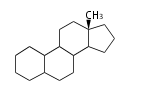
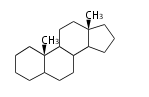
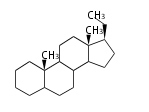
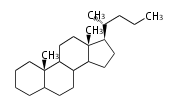
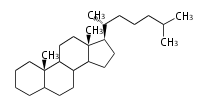
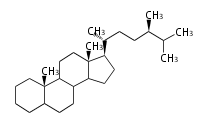
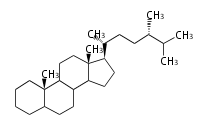
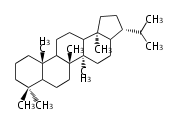
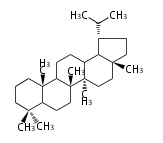
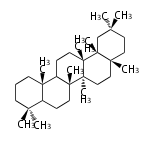
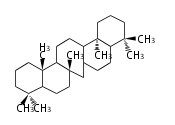
- y ... backbone structure (母核構造)
|
- r ... number of major rings (環構造数)
Click above categories to see details.
- h ... hydroxylation pattern (水酸基数)
Click above categories to see details.
- g ... glycosylation pattern(糖修飾パターン)
Click above categories to see details.
- n ... number of sugars (修飾糖数)
Click above categories to see details.
- c ... serial number (通し番号)
This category currently contains no pages or media.