Mol:FL63ACGS0003
From Metabolomics.JP
(Difference between revisions)
| Line 1: | Line 1: | ||
| − | + | ||
| − | + | ||
| − | Copyright: ARM project http://www.metabolome.jp/ | + | Copyright: ARM project http://www.metabolome.jp/ |
| − | 42 46 0 0 0 0 0 0 0 0999 V2000 | + | 42 46 0 0 0 0 0 0 0 0999 V2000 |
| − | -2.9009 0.8273 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -2.9009 0.8273 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -2.9009 0.2291 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -2.9009 0.2291 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -2.3829 -0.0699 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -2.3829 -0.0699 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -1.8649 0.2291 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -1.8649 0.2291 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -1.8649 0.8273 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -1.8649 0.8273 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -2.3829 1.1263 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -2.3829 1.1263 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -1.3469 -0.0699 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -1.3469 -0.0699 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -0.8289 0.2291 0.0000 C 0 0 1 0 0 0 0 0 0 0 0 0 | + | -0.8289 0.2291 0.0000 C 0 0 1 0 0 0 0 0 0 0 0 0 |
| − | -0.8289 0.8273 0.0000 C 0 0 2 0 0 0 0 0 0 0 0 0 | + | -0.8289 0.8273 0.0000 C 0 0 2 0 0 0 0 0 0 0 0 0 |
| − | -1.3469 1.1263 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | -1.3469 1.1263 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -0.3110 1.1263 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -0.3110 1.1263 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 0.2113 0.8248 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 0.2113 0.8248 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 0.7336 1.1263 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 0.7336 1.1263 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 0.7336 1.7294 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 0.7336 1.7294 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 0.2113 2.0310 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 0.2113 2.0310 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -0.3110 1.7294 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -0.3110 1.7294 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 1.2335 2.0180 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | 1.2335 2.0180 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -3.4189 1.1263 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | -3.4189 1.1263 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -0.3110 -0.0699 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | -0.3110 -0.0699 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -2.3829 -0.6667 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | -2.3829 -0.6667 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 0.2113 2.6332 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | 0.2113 2.6332 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -0.7965 -2.1604 0.0000 C 0 0 2 0 0 0 0 0 0 0 0 0 | + | -0.7965 -2.1604 0.0000 C 0 0 2 0 0 0 0 0 0 0 0 0 |
| − | -0.2144 -2.0102 0.0000 C 0 0 1 0 0 0 0 0 0 0 0 0 | + | -0.2144 -2.0102 0.0000 C 0 0 1 0 0 0 0 0 0 0 0 0 |
| − | -0.2422 -1.4155 0.0000 C 0 0 1 0 0 0 0 0 0 0 0 0 | + | -0.2422 -1.4155 0.0000 C 0 0 1 0 0 0 0 0 0 0 0 0 |
| − | 0.0485 -0.9023 0.0000 C 0 0 1 0 0 0 0 0 0 0 0 0 | + | 0.0485 -0.9023 0.0000 C 0 0 1 0 0 0 0 0 0 0 0 0 |
| − | -0.5900 -1.1276 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | -0.5900 -1.1276 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -0.5336 -1.7473 0.0000 C 0 0 3 0 0 0 0 0 0 0 0 0 | + | -0.5336 -1.7473 0.0000 C 0 0 3 0 0 0 0 0 0 0 0 0 |
| − | -0.7965 -2.6332 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | -0.7965 -2.6332 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 0.5715 -2.2208 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | 0.5715 -2.2208 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 0.2912 -1.4082 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | 0.2912 -1.4082 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 0.7617 -0.9377 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 0.7617 -0.9377 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 1.0051 -0.5901 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | 1.0051 -0.5901 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 1.3766 -1.2927 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 1.3766 -1.2927 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 1.8402 -1.0250 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 1.8402 -1.0250 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 2.3034 -1.2925 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 2.3034 -1.2925 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 2.3034 -1.9365 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 2.3034 -1.9365 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 2.8611 -2.2585 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 2.8611 -2.2585 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 3.4189 -1.9365 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 3.4189 -1.9365 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 3.4189 -1.2925 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 3.4189 -1.2925 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 2.8611 -0.9704 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 2.8611 -0.9704 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -1.1412 -1.3344 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -1.1412 -1.3344 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -2.0415 -1.7697 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | -2.0415 -1.7697 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 1 2 2 0 0 0 0 | + | 1 2 2 0 0 0 0 |
| − | 2 3 1 0 0 0 0 | + | 2 3 1 0 0 0 0 |
| − | 3 4 2 0 0 0 0 | + | 3 4 2 0 0 0 0 |
| − | 4 5 1 0 0 0 0 | + | 4 5 1 0 0 0 0 |
| − | 5 6 2 0 0 0 0 | + | 5 6 2 0 0 0 0 |
| − | 6 1 1 0 0 0 0 | + | 6 1 1 0 0 0 0 |
| − | 4 7 1 0 0 0 0 | + | 4 7 1 0 0 0 0 |
| − | 7 8 1 0 0 0 0 | + | 7 8 1 0 0 0 0 |
| − | 8 9 1 0 0 0 0 | + | 8 9 1 0 0 0 0 |
| − | 9 10 1 0 0 0 0 | + | 9 10 1 0 0 0 0 |
| − | 10 5 1 0 0 0 0 | + | 10 5 1 0 0 0 0 |
| − | 9 11 1 6 0 0 0 | + | 9 11 1 6 0 0 0 |
| − | 11 12 2 0 0 0 0 | + | 11 12 2 0 0 0 0 |
| − | 12 13 1 0 0 0 0 | + | 12 13 1 0 0 0 0 |
| − | 13 14 2 0 0 0 0 | + | 13 14 2 0 0 0 0 |
| − | 14 15 1 0 0 0 0 | + | 14 15 1 0 0 0 0 |
| − | 15 16 2 0 0 0 0 | + | 15 16 2 0 0 0 0 |
| − | 16 11 1 0 0 0 0 | + | 16 11 1 0 0 0 0 |
| − | 17 14 1 0 0 0 0 | + | 17 14 1 0 0 0 0 |
| − | 1 18 1 0 0 0 0 | + | 1 18 1 0 0 0 0 |
| − | 8 19 1 1 0 0 0 | + | 8 19 1 1 0 0 0 |
| − | 3 20 1 0 0 0 0 | + | 3 20 1 0 0 0 0 |
| − | 15 21 1 0 0 0 0 | + | 15 21 1 0 0 0 0 |
| − | 22 23 1 1 0 0 0 | + | 22 23 1 1 0 0 0 |
| − | 23 24 1 1 0 0 0 | + | 23 24 1 1 0 0 0 |
| − | 25 24 1 1 0 0 0 | + | 25 24 1 1 0 0 0 |
| − | 25 26 1 0 0 0 0 | + | 25 26 1 0 0 0 0 |
| − | 26 27 1 0 0 0 0 | + | 26 27 1 0 0 0 0 |
| − | 27 22 1 0 0 0 0 | + | 27 22 1 0 0 0 0 |
| − | 22 28 1 0 0 0 0 | + | 22 28 1 0 0 0 0 |
| − | 23 29 1 0 0 0 0 | + | 23 29 1 0 0 0 0 |
| − | 24 30 1 0 0 0 0 | + | 24 30 1 0 0 0 0 |
| − | 19 25 1 0 0 0 0 | + | 19 25 1 0 0 0 0 |
| − | 30 31 1 0 0 0 0 | + | 30 31 1 0 0 0 0 |
| − | 31 32 2 0 0 0 0 | + | 31 32 2 0 0 0 0 |
| − | 31 33 1 0 0 0 0 | + | 31 33 1 0 0 0 0 |
| − | 33 34 2 0 0 0 0 | + | 33 34 2 0 0 0 0 |
| − | 34 35 1 0 0 0 0 | + | 34 35 1 0 0 0 0 |
| − | 35 36 2 0 0 0 0 | + | 35 36 2 0 0 0 0 |
| − | 36 37 1 0 0 0 0 | + | 36 37 1 0 0 0 0 |
| − | 37 38 2 0 0 0 0 | + | 37 38 2 0 0 0 0 |
| − | 38 39 1 0 0 0 0 | + | 38 39 1 0 0 0 0 |
| − | 39 40 2 0 0 0 0 | + | 39 40 2 0 0 0 0 |
| − | 40 35 1 0 0 0 0 | + | 40 35 1 0 0 0 0 |
| − | 27 41 1 0 0 0 0 | + | 27 41 1 0 0 0 0 |
| − | 41 42 1 0 0 0 0 | + | 41 42 1 0 0 0 0 |
| − | M STY 1 1 SUP | + | M STY 1 1 SUP |
| − | M SLB 1 1 1 | + | M SLB 1 1 1 |
| − | M SAL 1 2 41 42 | + | M SAL 1 2 41 42 |
| − | M SBL 1 1 45 | + | M SBL 1 1 45 |
| − | M SMT 1 CH2OH | + | M SMT 1 CH2OH |
| − | M SVB 1 45 -1.1412 -1.3344 | + | M SVB 1 45 -1.1412 -1.3344 |
| − | S SKP 8 | + | S SKP 8 |
| − | ID FL63ACGS0003 | + | ID FL63ACGS0003 |
| − | KNApSAcK_ID C00008839 | + | KNApSAcK_ID C00008839 |
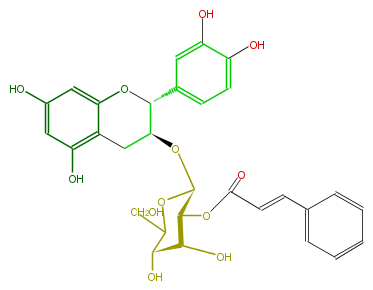
| − | NAME Epicatechin 3-O-(2-trans-cinnamoyl-beta-D-allopyranoside) | + | NAME Epicatechin 3-O-(2-trans-cinnamoyl-beta-D-allopyranoside) |
| − | CAS_RN 98752-08-0 | + | CAS_RN 98752-08-0 |
| − | FORMULA C30H30O12 | + | FORMULA C30H30O12 |
| − | EXACTMASS 582.173726424 | + | EXACTMASS 582.173726424 |
| − | AVERAGEMASS 582.552 | + | AVERAGEMASS 582.552 |
| − | SMILES Oc(c5)cc(O1)c(c5O)C[C@H](O[C@@H]([C@@H](OC(C=Cc(c4)cccc4)=O)3)OC(CO)[C@@H]([C@H](O)3)O)[C@H]1c(c2)ccc(O)c(O)2 | + | SMILES Oc(c5)cc(O1)c(c5O)C[C@H](O[C@@H]([C@@H](OC(C=Cc(c4)cccc4)=O)3)OC(CO)[C@@H]([C@H](O)3)O)[C@H]1c(c2)ccc(O)c(O)2 |
M END | M END | ||
| − | |||
Latest revision as of 09:00, 14 March 2009
Copyright: ARM project http://www.metabolome.jp/
42 46 0 0 0 0 0 0 0 0999 V2000
-2.9009 0.8273 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-2.9009 0.2291 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-2.3829 -0.0699 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-1.8649 0.2291 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-1.8649 0.8273 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-2.3829 1.1263 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-1.3469 -0.0699 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-0.8289 0.2291 0.0000 C 0 0 1 0 0 0 0 0 0 0 0 0
-0.8289 0.8273 0.0000 C 0 0 2 0 0 0 0 0 0 0 0 0
-1.3469 1.1263 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
-0.3110 1.1263 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
0.2113 0.8248 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
0.7336 1.1263 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
0.7336 1.7294 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
0.2113 2.0310 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-0.3110 1.7294 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
1.2335 2.0180 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
-3.4189 1.1263 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
-0.3110 -0.0699 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
-2.3829 -0.6667 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
0.2113 2.6332 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
-0.7965 -2.1604 0.0000 C 0 0 2 0 0 0 0 0 0 0 0 0
-0.2144 -2.0102 0.0000 C 0 0 1 0 0 0 0 0 0 0 0 0
-0.2422 -1.4155 0.0000 C 0 0 1 0 0 0 0 0 0 0 0 0
0.0485 -0.9023 0.0000 C 0 0 1 0 0 0 0 0 0 0 0 0
-0.5900 -1.1276 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
-0.5336 -1.7473 0.0000 C 0 0 3 0 0 0 0 0 0 0 0 0
-0.7965 -2.6332 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
0.5715 -2.2208 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
0.2912 -1.4082 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
0.7617 -0.9377 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
1.0051 -0.5901 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
1.3766 -1.2927 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
1.8402 -1.0250 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
2.3034 -1.2925 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
2.3034 -1.9365 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
2.8611 -2.2585 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
3.4189 -1.9365 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
3.4189 -1.2925 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
2.8611 -0.9704 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-1.1412 -1.3344 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-2.0415 -1.7697 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
1 2 2 0 0 0 0
2 3 1 0 0 0 0
3 4 2 0 0 0 0
4 5 1 0 0 0 0
5 6 2 0 0 0 0
6 1 1 0 0 0 0
4 7 1 0 0 0 0
7 8 1 0 0 0 0
8 9 1 0 0 0 0
9 10 1 0 0 0 0
10 5 1 0 0 0 0
9 11 1 6 0 0 0
11 12 2 0 0 0 0
12 13 1 0 0 0 0
13 14 2 0 0 0 0
14 15 1 0 0 0 0
15 16 2 0 0 0 0
16 11 1 0 0 0 0
17 14 1 0 0 0 0
1 18 1 0 0 0 0
8 19 1 1 0 0 0
3 20 1 0 0 0 0
15 21 1 0 0 0 0
22 23 1 1 0 0 0
23 24 1 1 0 0 0
25 24 1 1 0 0 0
25 26 1 0 0 0 0
26 27 1 0 0 0 0
27 22 1 0 0 0 0
22 28 1 0 0 0 0
23 29 1 0 0 0 0
24 30 1 0 0 0 0
19 25 1 0 0 0 0
30 31 1 0 0 0 0
31 32 2 0 0 0 0
31 33 1 0 0 0 0
33 34 2 0 0 0 0
34 35 1 0 0 0 0
35 36 2 0 0 0 0
36 37 1 0 0 0 0
37 38 2 0 0 0 0
38 39 1 0 0 0 0
39 40 2 0 0 0 0
40 35 1 0 0 0 0
27 41 1 0 0 0 0
41 42 1 0 0 0 0
M STY 1 1 SUP
M SLB 1 1 1
M SAL 1 2 41 42
M SBL 1 1 45
M SMT 1 CH2OH
M SVB 1 45 -1.1412 -1.3344
S SKP 8
ID FL63ACGS0003
KNApSAcK_ID C00008839
NAME Epicatechin 3-O-(2-trans-cinnamoyl-beta-D-allopyranoside)
CAS_RN 98752-08-0
FORMULA C30H30O12
EXACTMASS 582.173726424
AVERAGEMASS 582.552
SMILES Oc(c5)cc(O1)c(c5O)C[C@H](O[C@@H]([C@@H](OC(C=Cc(c4)cccc4)=O)3)OC(CO)[C@@H]([C@H](O)3)O)[C@H]1c(c2)ccc(O)c(O)2
M END