Mol:FL3FRNNF0006
From Metabolomics.JP
(Difference between revisions)
| Line 1: | Line 1: | ||
| − | + | ||
| − | + | ||
| − | Copyright: ARM project http://www.metabolome.jp/ | + | Copyright: ARM project http://www.metabolome.jp/ |
| − | 38 42 0 0 0 0 0 0 0 0999 V2000 | + | 38 42 0 0 0 0 0 0 0 0999 V2000 |
| − | 0.0314 -0.8723 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 0.0314 -0.8723 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 0.0361 -0.0473 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 0.0361 -0.0473 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 0.7412 -1.2889 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 0.7412 -1.2889 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 1.4604 -0.8804 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 1.4604 -0.8804 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 1.4650 -0.0554 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 1.4650 -0.0554 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 0.7529 0.3611 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | 0.7529 0.3611 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 2.8940 -0.0635 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 2.8940 -0.0635 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 2.1819 0.3530 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 2.1819 0.3530 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -0.6737 0.3692 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -0.6737 0.3692 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -1.3928 -0.0392 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -1.3928 -0.0392 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -1.3975 -0.8642 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -1.3975 -0.8642 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -0.6877 -1.2807 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -0.6877 -1.2807 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 3.6030 0.3579 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 3.6030 0.3579 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 3.6002 1.1956 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 3.6002 1.1956 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 2.8879 1.6125 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 2.8879 1.6125 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 2.1788 1.1910 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 2.1788 1.1910 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -0.6877 -2.1057 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | -0.6877 -2.1057 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 0.7412 -2.1138 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | 0.7412 -2.1138 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 4.3147 1.6081 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | 4.3147 1.6081 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 1.8418 -1.4322 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 1.8418 -1.4322 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 2.5681 -1.4155 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 2.5681 -1.4155 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 2.8978 -0.8712 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | 2.8978 -0.8712 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 2.9806 -2.1300 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 2.9806 -2.1300 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 2.2664 -2.5423 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 2.2664 -2.5423 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 3.6953 -1.7173 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | 3.6953 -1.7173 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 3.3931 -2.8445 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | 3.3931 -2.8445 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -2.1760 0.2202 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | -2.1760 0.2202 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -2.6647 -0.4437 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -2.6647 -0.4437 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -2.1760 -1.1147 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -2.1760 -1.1147 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -0.6737 1.1942 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -0.6737 1.1942 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -1.3864 1.6057 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -1.3864 1.6057 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -1.3864 2.4317 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -1.3864 2.4317 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -2.1004 2.8440 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -2.1004 2.8440 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -0.6715 2.8445 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -0.6715 2.8445 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -3.4897 -0.4437 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -3.4897 -0.4437 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -3.4897 -1.2679 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 | + | -3.4897 -1.2679 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -4.3147 -0.4437 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -4.3147 -0.4437 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | -3.4897 0.3821 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 | + | -3.4897 0.3821 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0 |
| − | 1 2 1 0 0 0 0 | + | 1 2 1 0 0 0 0 |
| − | 1 3 1 0 0 0 0 | + | 1 3 1 0 0 0 0 |
| − | 3 4 1 0 0 0 0 | + | 3 4 1 0 0 0 0 |
| − | 4 5 2 0 0 0 0 | + | 4 5 2 0 0 0 0 |
| − | 5 6 1 0 0 0 0 | + | 5 6 1 0 0 0 0 |
| − | 6 2 1 0 0 0 0 | + | 6 2 1 0 0 0 0 |
| − | 7 8 1 0 0 0 0 | + | 7 8 1 0 0 0 0 |
| − | 8 5 1 0 0 0 0 | + | 8 5 1 0 0 0 0 |
| − | 2 9 2 0 0 0 0 | + | 2 9 2 0 0 0 0 |
| − | 9 10 1 0 0 0 0 | + | 9 10 1 0 0 0 0 |
| − | 10 11 2 0 0 0 0 | + | 10 11 2 0 0 0 0 |
| − | 11 12 1 0 0 0 0 | + | 11 12 1 0 0 0 0 |
| − | 12 1 2 0 0 0 0 | + | 12 1 2 0 0 0 0 |
| − | 7 13 2 0 0 0 0 | + | 7 13 2 0 0 0 0 |
| − | 13 14 1 0 0 0 0 | + | 13 14 1 0 0 0 0 |
| − | 14 15 2 0 0 0 0 | + | 14 15 2 0 0 0 0 |
| − | 15 16 1 0 0 0 0 | + | 15 16 1 0 0 0 0 |
| − | 16 8 2 0 0 0 0 | + | 16 8 2 0 0 0 0 |
| − | 12 17 1 0 0 0 0 | + | 12 17 1 0 0 0 0 |
| − | 3 18 2 0 0 0 0 | + | 3 18 2 0 0 0 0 |
| − | 14 19 1 0 0 0 0 | + | 14 19 1 0 0 0 0 |
| − | 4 20 1 0 0 0 0 | + | 4 20 1 0 0 0 0 |
| − | 20 21 1 0 0 0 0 | + | 20 21 1 0 0 0 0 |
| − | 21 22 1 0 0 0 0 | + | 21 22 1 0 0 0 0 |
| − | 22 7 1 0 0 0 0 | + | 22 7 1 0 0 0 0 |
| − | 21 23 1 0 0 0 0 | + | 21 23 1 0 0 0 0 |
| − | 23 24 1 0 0 0 0 | + | 23 24 1 0 0 0 0 |
| − | 23 25 1 0 0 0 0 | + | 23 25 1 0 0 0 0 |
| − | 23 26 1 0 0 0 0 | + | 23 26 1 0 0 0 0 |
| − | 10 27 1 0 0 0 0 | + | 10 27 1 0 0 0 0 |
| − | 27 28 1 0 0 0 0 | + | 27 28 1 0 0 0 0 |
| − | 28 29 1 0 0 0 0 | + | 28 29 1 0 0 0 0 |
| − | 29 11 1 0 0 0 0 | + | 29 11 1 0 0 0 0 |
| − | 9 30 1 0 0 0 0 | + | 9 30 1 0 0 0 0 |
| − | 30 31 1 0 0 0 0 | + | 30 31 1 0 0 0 0 |
| − | 31 32 2 0 0 0 0 | + | 31 32 2 0 0 0 0 |
| − | 32 33 1 0 0 0 0 | + | 32 33 1 0 0 0 0 |
| − | 32 34 1 0 0 0 0 | + | 32 34 1 0 0 0 0 |
| − | 28 35 1 0 0 0 0 | + | 28 35 1 0 0 0 0 |
| − | 35 36 1 0 0 0 0 | + | 35 36 1 0 0 0 0 |
| − | 35 37 1 0 0 0 0 | + | 35 37 1 0 0 0 0 |
| − | 35 38 1 0 0 0 0 | + | 35 38 1 0 0 0 0 |
| − | S SKP 8 | + | S SKP 8 |
| − | ID FL3FRNNF0006 | + | ID FL3FRNNF0006 |
| − | KNApSAcK_ID C00013498 | + | KNApSAcK_ID C00013498 |
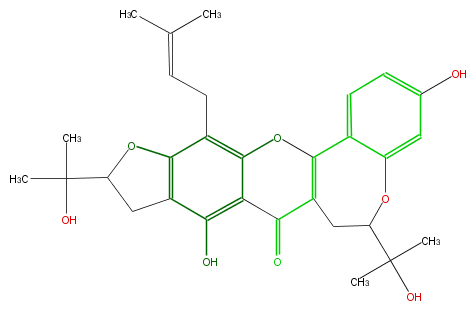
| − | NAME Carpelastofuran;6,7,10,11-Tetrahydro-3,9-dihydroxy-6,11-bis(1-hydroxy-1-methylethyl)-13-(3-methyl-2-butenyl)-8H-furo[3',2':6,7][1]benzopyrano[3,2-d][1]benzoxepin-8-one | + | NAME Carpelastofuran;6,7,10,11-Tetrahydro-3,9-dihydroxy-6,11-bis(1-hydroxy-1-methylethyl)-13-(3-methyl-2-butenyl)-8H-furo[3',2':6,7][1]benzopyrano[3,2-d][1]benzoxepin-8-one |
| − | CAS_RN 404889-57-2 | + | CAS_RN 404889-57-2 |
| − | FORMULA C30H34O8 | + | FORMULA C30H34O8 |
| − | EXACTMASS 522.225368064 | + | EXACTMASS 522.225368064 |
| − | AVERAGEMASS 522.5861600000001 | + | AVERAGEMASS 522.5861600000001 |
| − | SMILES Oc(c52)c(C1)c(c(CC=C(C)C)c2OC(=C(C(=O)5)3)c(c4)c(cc(O)c4)OC(C(C)(C)O)C3)OC(C(C)(C)O)1 | + | SMILES Oc(c52)c(C1)c(c(CC=C(C)C)c2OC(=C(C(=O)5)3)c(c4)c(cc(O)c4)OC(C(C)(C)O)C3)OC(C(C)(C)O)1 |
M END | M END | ||
| − | |||
Latest revision as of 09:00, 14 March 2009
Copyright: ARM project http://www.metabolome.jp/
38 42 0 0 0 0 0 0 0 0999 V2000
0.0314 -0.8723 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
0.0361 -0.0473 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
0.7412 -1.2889 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
1.4604 -0.8804 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
1.4650 -0.0554 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
0.7529 0.3611 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
2.8940 -0.0635 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
2.1819 0.3530 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-0.6737 0.3692 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-1.3928 -0.0392 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-1.3975 -0.8642 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-0.6877 -1.2807 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
3.6030 0.3579 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
3.6002 1.1956 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
2.8879 1.6125 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
2.1788 1.1910 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-0.6877 -2.1057 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
0.7412 -2.1138 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
4.3147 1.6081 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
1.8418 -1.4322 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
2.5681 -1.4155 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
2.8978 -0.8712 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
2.9806 -2.1300 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
2.2664 -2.5423 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
3.6953 -1.7173 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
3.3931 -2.8445 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
-2.1760 0.2202 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
-2.6647 -0.4437 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-2.1760 -1.1147 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-0.6737 1.1942 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-1.3864 1.6057 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-1.3864 2.4317 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-2.1004 2.8440 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-0.6715 2.8445 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-3.4897 -0.4437 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-3.4897 -1.2679 0.0000 O 0 0 0 0 0 0 0 0 0 0 0 0
-4.3147 -0.4437 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
-3.4897 0.3821 0.0000 C 0 0 0 0 0 0 0 0 0 0 0 0
1 2 1 0 0 0 0
1 3 1 0 0 0 0
3 4 1 0 0 0 0
4 5 2 0 0 0 0
5 6 1 0 0 0 0
6 2 1 0 0 0 0
7 8 1 0 0 0 0
8 5 1 0 0 0 0
2 9 2 0 0 0 0
9 10 1 0 0 0 0
10 11 2 0 0 0 0
11 12 1 0 0 0 0
12 1 2 0 0 0 0
7 13 2 0 0 0 0
13 14 1 0 0 0 0
14 15 2 0 0 0 0
15 16 1 0 0 0 0
16 8 2 0 0 0 0
12 17 1 0 0 0 0
3 18 2 0 0 0 0
14 19 1 0 0 0 0
4 20 1 0 0 0 0
20 21 1 0 0 0 0
21 22 1 0 0 0 0
22 7 1 0 0 0 0
21 23 1 0 0 0 0
23 24 1 0 0 0 0
23 25 1 0 0 0 0
23 26 1 0 0 0 0
10 27 1 0 0 0 0
27 28 1 0 0 0 0
28 29 1 0 0 0 0
29 11 1 0 0 0 0
9 30 1 0 0 0 0
30 31 1 0 0 0 0
31 32 2 0 0 0 0
32 33 1 0 0 0 0
32 34 1 0 0 0 0
28 35 1 0 0 0 0
35 36 1 0 0 0 0
35 37 1 0 0 0 0
35 38 1 0 0 0 0
S SKP 8
ID FL3FRNNF0006
KNApSAcK_ID C00013498
NAME Carpelastofuran;6,7,10,11-Tetrahydro-3,9-dihydroxy-6,11-bis(1-hydroxy-1-methylethyl)-13-(3-methyl-2-butenyl)-8H-furo[3',2':6,7][1]benzopyrano[3,2-d][1]benzoxepin-8-one
CAS_RN 404889-57-2
FORMULA C30H34O8
EXACTMASS 522.225368064
AVERAGEMASS 522.5861600000001
SMILES Oc(c52)c(C1)c(c(CC=C(C)C)c2OC(=C(C(=O)5)3)c(c4)c(cc(O)c4)OC(C(C)(C)O)C3)OC(C(C)(C)O)1
M END