Doc:FL63AC
From Metabolomics.JP
(Difference between revisions)
m |
|||
| Line 1: | Line 1: | ||
{{Twocolumn| | {{Twocolumn| | ||
| − | Catechins refer to a subgroup of [[:Category:FL63| | + | Catechins refer to a subgroup of [[:Category:FL63|flavan 3-ol]] derivatives (FL63AC). |
| − | [[FL63ACNS0002|(-)-Epicatechin]] | + | The two chiral center at C2 and C3 of the flavan 3-ols produces 4 isomers, |
| + | and [[FL63ACNS0001|(+)-Catechin]] and its stereoisomer | ||
| + | [[FL63ACNS0002|(-)-Epicatechin]] are naturally abundant. Less abundant are [[FL63ACNS0004|(-)-Catechin]] and [[FL63ACNS0003|(+)-Epicatechin]]. | ||
| + | Gallocatechins are their hydrolized forms. | ||
| | | | ||
| − | いわゆるカテキン類とは、[[:Category:FL63|Flavan 3-ol]] | + | いわゆるカテキン類とは、[[:Category:FL63|Flavan 3-ol]]の下にあるグループを指します。C2, C3位にある不斉炭素によって4つの異性体が作られますが、自然界に豊富なのは [[FL63ACNS0001|(+)-カテキン]] とその立体異性体である |
| − | [[FL63ACNS0002|(-)- | + | [[FL63ACNS0002|(-)-エピカテキン]] です。 [[FL63ACNS0004|(-)-カテキン]] と [[FL63ACNS0003|(+)-エピカテキン]] は自然界にあまりみられません。 |
| + | これらの分子が水酸化されたものを、ガロカテキンと呼びます。 | ||
}} | }} | ||
| Line 34: | Line 38: | ||
|} | |} | ||
| + | {{Twocolumn| | ||
Among the stereoisomers, the bioavailability in human follows the order: | Among the stereoisomers, the bioavailability in human follows the order: | ||
| + | | | ||
| + | 立体異性体の中で、ヒトにおける利用活性は以下の順番になります。 | ||
| + | }} | ||
| + | <center> | ||
{| | {| | ||
| − | |(-)-epicatechin ||>|| (+)-catechin ||=|| (+)-epicatechin ||>|| (-)-catechin | + | | (-)-epicatechin ||>|| (+)-catechin ||=|| (+)-epicatechin ||>|| (-)-catechin |
|} | |} | ||
| − | The circulation level of (-)-epicatechin is 6 times higher than that of (-)-catechin. | + | </center> |
| + | {{Twocolumn| | ||
| + | The circulation level of (-)-epicatechin is 6 times higher than that of (-)-catechin. Naturally abundant species are more bioavailable than less abundant ones. | ||
| + | | | ||
| + | (-)-エピカテキンが体内に入る効率は(-)-カテキンの6倍にもなり、天然に多く産する分子種が多く吸収されることがわかります。 | ||
| + | }} | ||
Revision as of 22:08, 18 August 2010
Catechins refer to a subgroup of flavan 3-ol derivatives (FL63AC). The two chiral center at C2 and C3 of the flavan 3-ols produces 4 isomers, and (+)-Catechin and its stereoisomer (-)-Epicatechin are naturally abundant. Less abundant are (-)-Catechin and (+)-Epicatechin. Gallocatechins are their hydrolized forms.
| Structure | 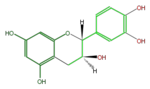
|
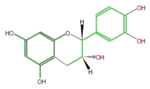
|
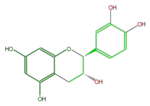
|
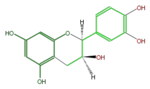
|
|---|---|---|---|---|
| Name | (+)-Catechin or D-Catechin |
(-)-Epicatechin or L-Epicatechin |
ent-Catechin or (-)-Catechin |
ent-Epicatechin or (+)-Epicatechin |
| B-ring stereo | ↓ | ↓ | ↑ | ↑ |
| 3-Hydroxyl stereo | ↑ | ↓ | ↓ | ↑ |
| Gallocatechins | (+)-Gallocatechin | (-)-Epigallocatechin | (-)-Gallocatechin |
Among the stereoisomers, the bioavailability in human follows the order:
| (-)-epicatechin | > | (+)-catechin | = | (+)-epicatechin | > | (-)-catechin |
The circulation level of (-)-epicatechin is 6 times higher than that of (-)-catechin. Naturally abundant species are more bioavailable than less abundant ones.