FL63ACNS0002
From Metabolomics.JP
| Flavonoid Top | Molecule Index | Author Index | Journals | Structure Search | Food | New Input |
Upper classes : FL Flavonoid : FL6 Flavan : FL63 Flavan 3-ol : FL63AC Catechin and Epicatechin (75 pages) : FL63ACNS Simple substitution (24 pages)
Catechins refer to a subgroup of flavan 3-ol derivatives (FL63AC). The two chiral center at C2 and C3 of the flavan 3-ols produces 4 isomers, and (+)-Catechin and its stereoisomer (-)-Epicatechin are naturally abundant. Less abundant are (-)-Catechin and (+)-Epicatechin.
- afzelechin ... catechin minus 1 hydroxyl group in ring B
- gallocatechin ... catechin plus 1 hydroxyl group in ring B
| Structure | 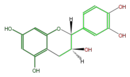
|
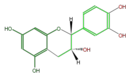
|
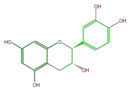
|
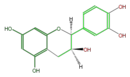
|
|---|---|---|---|---|
| Name | (+)-Catechin or D-Catechin |
(-)-Epicatechin or L-Epicatechin |
ent-Catechin or (-)-Catechin |
ent-Epicatechin or (+)-Epicatechin |
| B-ring stereo | ↓ | ↓ | ↑ | ↑ |
| 3-Hydroxyl stereo | ↑ | ↓ | ↓ | ↑ |
| Afzelechins | (+)-Afzelechin | (-)-Epiafzelechin | ent-Epiafzelechin | |
| Gallocatechins | (+)-Gallocatechin | (-)-Epigallocatechin | ent-Gallocatechin |
Among the stereoisomers, the bioavailability in human follows the order:
| (-)-epicatechin | > | (+)-catechin | = | (+)-epicatechin | > | (-)-catechin |
The circulation level of (-)-epicatechin is 6 times higher than that of (-)-catechin. Naturally abundant species are more bioavailable than less abundant ones.
| IDs and Links | |
|---|---|
| LipidBank | [1] |
| LipidMaps | [2] |
| CAS | 490-46-0 |
| KEGG | {{{KEGG}}} |
| KNApSAcK | |
| CDX file | |
| MOL file | FL63ACNS0002.mol |
| (-)-Epicatechin | |
|---|---|
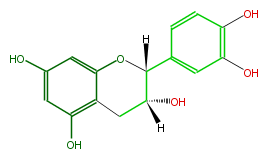
| |
| Structural Information | |
| Systematic Name | (2R) -2alpha- (3,4-Dihydroxyphenyl) -3,4-dihydro-2H-1-benzopyran-3alpha,5,7-triol |
| Common Name |
|
| Symbol | |
| Formula | C15H14O6 |
| Exact Mass | 290.07903818 |
| Average Mass | 290.26806 |
| SMILES | Oc(c3)cc(O1)c(c(O)3)CC([H])(O)C([H])1c(c2)cc(O)c(O |
| Physicochemical Information | |
| Melting Point | |
| Boiling Point | |
| Density | |
| Optical Rotation | |
| Reflactive Index | |
| Solubility | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | |
| Chromatograms | |