Category:FL
m |
(→{{Bilingual|フラボノイド一般|General Flavonoid}}) |
||
| Line 402: | Line 402: | ||
* [[Doc:Tea/Health|Tea and health]] 茶と健康 | * [[Doc:Tea/Health|Tea and health]] 茶と健康 | ||
| − | + | ;{{Bilingual|抗紫外、抗酸化作用|Photoprotectant, anti-oxidant activity}} | |
{{Twocolumn| | {{Twocolumn| | ||
| − | + | Not only anthocyanins but even simple structures such as chalcone can absorb UV wavelengths strongly. The ability of photoprotection is derived from its anti-oxidative activity. | |
| − | + | ||
| − | Not only anthocyanins but even simple structures such as chalcone can absorb UV wavelengths strongly. The ability of photoprotection is derived from its anti-oxidative activity. | + | |
| − | + | ||
| | | | ||
| − | + | アントシアニンだけでなく、カルコンのような構造の簡単なフラボノイドでも紫外線をよく吸収します。効紫外線効果は効酸化作用に関係しています。 | |
| − | + | ||
| − | + | ||
}} | }} | ||
| − | + | [[Doc:Antioxidant|{{Bilingual|抗酸化作用の詳細|Details of antioxidant activity}}]] | |
| − | + | ;{{Bilingual|血圧効果作用|Hypotensive activity}} | |
| − | + | {{Twocolumn| | |
| − | {{Twocolumn | + | |
| − | | | + | |
Quercetin in onions and flavanols in cocoa are said to reduce blood pressure in hypertensive animals <ref>Hooper L, Kroon PA, Rimm EB, et al. (2008) "Flavonoids, flavonoid-rich foods, and cardiovascular risk: a meta-analysis of randomized controlled trials" Am J Clin Nutr 88:38–50 PMID 18614722</ref>, but their effect or mechanism is not clinically conclusive. | Quercetin in onions and flavanols in cocoa are said to reduce blood pressure in hypertensive animals <ref>Hooper L, Kroon PA, Rimm EB, et al. (2008) "Flavonoids, flavonoid-rich foods, and cardiovascular risk: a meta-analysis of randomized controlled trials" Am J Clin Nutr 88:38–50 PMID 18614722</ref>, but their effect or mechanism is not clinically conclusive. | ||
| − | | | + | | |
玉葱のクエルセチンやココアのフラバノールは血圧降下作用があるといわれますが、その効果や医学的メカニズムははっきりしていません。 | 玉葱のクエルセチンやココアのフラバノールは血圧降下作用があるといわれますが、その効果や医学的メカニズムははっきりしていません。 | ||
}} | }} | ||
| − | |||
<references/> | <references/> | ||
| − | {{ | + | ;{{Bilingual|抗菌、抗カビ作用|Anti-bacteria, anti-fungal activity}} |
| − | | | + | {{Twocolumn| |
| − | + | Tannins (proanthocyanidins) show anti-bacterial activity. | |
| − | | | + | | |
| − | + | タンニン (プロアントシアニジニン) は一般に抗菌、抗カビ作用があります。 | |
}} | }} | ||
Revision as of 15:21, 30 September 2010
Flavonoid
| Flavonoid Top | Molecule Index | Author Index | Journals | Structure Search | Food | New Input |
Contents |
Class Overview
The word "flavonoid" comes from its Latin origin flavus (yellow) with oid, meaning yellow-ish. It comes from its history as yellow natural dye (quercetin and kaempferol are the most widespread flavone dyes. See flavone).
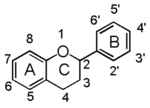
Chemically speaking, it is a class of plant secondary metabolites that have two benzene rings (each called A-ring and B-ring) connected by a chain of three carbons (Figure 1).
The carbon chain, corresponding to the numbers 2,3,4 in Figure 1, is linked to a hydroxyl group in the A-ring to form the C-ring. The class of flavonoids are usually determined by the modification pattern of the C-ring (Table 1).
Flavonoid is utilized in many industrial processes from pigments to food additives. Often heard names include anthocyanin, catechin, flavan, and isoflavone.
Biosynthesis
Flavonoid is synthesized through both the phenylpropanoid-acetate pathway and the acetate-malonate pathway in all higher plants (but not algae). Most plants contain the 7 major subgroups (chalcones, flavanones, flavones, flavonols, flavans, anthocyanidins, and anthycyanins), but aurones or isoflavonoids are not ubiquitous.
| 4-coumaroyl CoA + malonyl CoA | ||||||||||||||||
| | ||||||||||||||||
| CHALCONES, AURONES |
chalconaringenin |
FLAVONES |
apigenin |
luteolin | ||||||||||||
| CHI |
| |||||||||||||||
IFS |
naringenin FLAVANONE |
kaempferol |
FLAVONOLS |
quercetin |
myricetin | |||||||||||
| ISO-FLAVONES |
F3H |
|||||||||||||||
| DIHYDRO FLAVONOLS |
dihydro-kaempferol |
F3'H +OH in B-ring |
dihydro-quercetin |
F3'5'H +OH in B-ring |
dihydro-myricetin | |||||||||||
| DFR |
|
|
||||||||||||||
| LEUCOANTHO-CYANIDINS |
leuco-pelargonidin |
|
leuco-cyanidin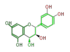
|
leuco-delphinidin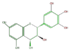
|
||||||||||||
| LDOX |
PROANTHO- CYANIDINS * | |
PROANTHO- CYANIDINS * | |
*...polymers of flavanols | |||||||||||
| ANTHO-CYANIDINS |
pelargonidin |
|
cyanidin |
|
delphinidin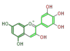
|
| ||||||||||
| UF3GT |
epi-afzelechin |
|
epi-catechin |
|
epigallo-catechin |
FLAVAN 3-OLS | ||||||||||
| ANTHO-CYANINS |
pelargonidin 3-glucoside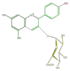
|
cyanidin 3-glucoside |
delphinidin 3-glucoside | |||||||||||||
|
|
| ||||||||||||||
| Information for the Above Abbreviated Gene Names | |||
|---|---|---|---|
| Six Structural Genes | |||
| Abbrev. | Name | Origin | Information |
| CHS | chalcone synthase | Bacterial polyketide synthases, particularly those in fatty acid synthesis (Verwoert et al. 1992) | Early response against light [1] [2] |
| CHI | chalcone-flavanone isomerase | Unclear and unique to plants.[3]
Eubacterium ramulus has the CHI activity. [4] |
Early response against light. |
| F3H | flavanone 3-hydroxylase | 2-oxoglutarate-dependent dioxygenase family [5] | Early response in Arabidopsis but late in Antirrhinum [6] |
| FLS | flavonol synthase | 2-oxoglutarate-dependent dioxygenase family [7] | Early response against light. In Arabidopsis, all structural genes are single-copy except for this one, to which six genes exist and two of them are not expressed. |
| DFR | dihydroflavonol 4-reductase | NADPH-dependent reductase associated with steroid metabolism [8] | Later response |
| ANS/LDOX | anthocyanidin synthase or leucoanthocyanidin dioxygenase | 2-oxoglutarate-dependent dioxygenase family | Later response |
| Auxiliary Genes | |||
| F3'H | flavonoid 3'-hydroxylase | cytochrome P450 hydroxylase family [9] | |
| F3'5'H | flavonoid 3',5'-hydroxylase | cytochrome P450 hydroxylase family [10] | Not reported in mosses or liverworts. The transformation of the F3'5'H and the cytochrome b5 gene of petunia into carnation changed its flower color deep purple.[11][12] |
| FSI | flavone synthase | Dioxygenase in parsley (FSI) and P450 monooxygenase in snapdragon (FSII). | |
| LAR (or LCR) | leucoanthocyanidin reductase | ||
| UF3GT | UDP flavonoid glucosyltransferase | ||
| GST | glutathione-S-transferase | Transport of flavonoids from cytoplasm to vacuole or cell walls requires both GST and the glutathione pump in ATP-binding cassette family.[13][14] | |
| AOMT | anthocyanin O-methyl transferase | ||
| |||
Bioactivity
Tea Related
- Tea category 茶の種類
- Tea consumption 茶の消費量
- Tea and health 茶と健康
- Photoprotectant, anti-oxidant activity
Not only anthocyanins but even simple structures such as chalcone can absorb UV wavelengths strongly. The ability of photoprotection is derived from its anti-oxidative activity.
Details of antioxidant activity
- Hypotensive activity
Quercetin in onions and flavanols in cocoa are said to reduce blood pressure in hypertensive animals [1], but their effect or mechanism is not clinically conclusive.
- ↑ Hooper L, Kroon PA, Rimm EB, et al. (2008) "Flavonoids, flavonoid-rich foods, and cardiovascular risk: a meta-analysis of randomized controlled trials" Am J Clin Nutr 88:38–50 PMID 18614722
- Anti-bacteria, anti-fungal activity
Tannins (proanthocyanidins) show anti-bacterial activity.
Bioavailability
| Flavonoid | Dose | Observation |
|---|---|---|
| catechin[1] | 5.8 g | 26% was excreted within 24h. m-hydroxyphenyl propionic acid was detected in plasma after 6h |
| 3-O-methyl-catechin[2] | 2 g | plasma level 11-18 ug/ml within 2h; 38% was excreted as glucuronides and sulphates in urine within 120h |
| quercetin[3] | 64 mg (as fried onion) | plasma level 1 uM, 2h later |
| quercetin[4] | 4 g (as supplement) | undetected in urine or plasma |
| decaffeinated green tea[5] | 88 mg EGCG, 82 mg EGC, 33 mg ECG, and 32 mg EC | plasma level 46-268 ng/ml, 82-206 ng/ml, undetected, and 40-80 ng/ml, respectively. |
- Isoflavones
Isoflavones are efficiently absorbed from the colon and exhibit the highest bioavailability. (Usually polyphenols absorbed from the colon show very low availability.) Daidzein and genistein are known to form chlorinated products (e.g. 3- and 8-chlorodaidzein), then are conjugated with glucuronides and excreted in bile.
- Anthocyanins
Although anthocyanins comprise ca. 50% of total polyphenols, they are poorly available (less than 1% of intake in urinary levels). Anthocyanins are absorbed from the stomach, and their glycosides appear in plasma soon after the intake. In blood, only anthocyanins exist in a non-conjugated form.
- Flavan
Flavan 3-ols and phenolic acids are efficiently abosrbed from the small instestine, a few hours after intake.
- Others
Non-absorbed flavonoids are transported to the colon, and subjected to metabolism by microbiota. These flavonoids are therefore absorbed much less compared to flavan 3-ols and phenolic acids. Esterification of phenolic acids (e.g. chlorogenic acid), however, reduces absorption.
During absorption, polyphenols are metabolized to form β-glucuronide and sulfate conjugates (phase II conjugation in the intestinal wall), and catechol units are methylated. C-glycosides such as puerarin remain stable and not conjugated. These metabolized forms show markedly different bioactivities from their aglycones.
- ↑ Das NP (1971) Biochem Pharmacol 20, 3435-3445
- ↑ Hackett AM, Griffiths LA, Wermeille M (1985) Xenobiotica 15, 907-914
- ↑ Hollman PCH et al (1995) Am J Clin Nutr 62, 1276-1282; Hollman PCH et al (1996) Free Rad Biol Med 21, 703-707
- ↑ Gugler R, Leshik M, Dengler HV (1975) Eur J Clin Pharmacol 9, 229-234
- ↑ Lee MT et al (1995) Cancer Epidemiol Biomarkers Prev 41,393-399
Database statistics/ranking
This database collects original references that report identification of flavonoid in various plant species. The database consists of three major namespaces: (flavonoid) compounds, plant species, and references. Currently, 6961 flavonoid structures, 3961 plant species, and 5215 references describing total 19861 metabolite-species relationships are registered.
| class | FL1 (chalcones) |
FL2 (flavanones) |
FL3 (flavones) |
FL4 (dihydroflavonols) |
FL5 (flavonols) |
FL6 (flavans) |
FL7 (anthocyanidins) |
FLI (isoflavonoids) |
FLN (neoflavonoids) |
Total |
|---|---|---|---|---|---|---|---|---|---|---|
| #data | 690 | 705 | 1479 | 272 | 1943 | 291 | 574 | 916 | 96 | 6961 |
Flavonoid content in food
For details, please visit this page.
- Food containing high Flavanone
Flavanones are rich in citrus, not in vegetables.
| Food | Flavanone (mg/100g) |
食品名 |
|---|---|---|
| grapefruit, raw or juice | 14-53 | グレープフルーツ (生、ジュース) |
| orange and tangerine, raw or juice | 13-33 | オレンジ、みかん (生、ジュース) |
- Food containing high flavone
Many herbs contain flavones. Parsley is rich in apigenin; celery and thyme in luteolin.
| Food | Flavone (mg/100g) |
食品名 |
|---|---|---|
| celery hearts, raw | 19 | セロリの芯 (生) |
| parsley, raw | 302 | パセリ (生) |
- Food containing high flavonol
Flavonols are prevalent in vegetables, usually in small amounts. Onions, kales, hot peppers are good sources.
| Food | Flavonol (mg/100g) |
食品名 |
|---|---|---|
| buckwheat | 23 | そば |
| cranberry, juice | 16 | クランベリー (ジュース) |
| onion, raw or boiled | 5-20 | たまねぎ (生、ゆで等) |
| kale, raw or canned | 18-34 | ケール (生、かんづめ) |
- Food containing high flavan
Catechins and epicatechins are contained in legumes and teas, but not in other vegetables.
| Food | Flavan (mg/100g or 100ml) |
食品名 |
|---|---|---|
| broadbeans, raw | ~50 | そらまめ (生) |
| dark chocolate bar | ~50 | ダークチョコレート |
| black grapes | 18 | 黒いブドウ |
| brewed black tea | > 16 | 淹れた紅茶 |
| brewed oolong tea | 50 | 淹れたウーロン茶 |
| brewed green tea | > 50 | 淹れた緑茶 |
- Food containing high anthocyanin
Anthocyanins are contained in berries. Vegetables supply only small amounts.
| Food | Anthocyanin (mg/100g) |
食品名 |
|---|---|---|
| blueberries, raw | 113 | ブルーベリー (生) |
| sweet cherries, raw | 116 | さくらんぼ (生) |
| elderberries, raw | 749 | エルダーベリー (生) |
| raspberries, raw | 49 | ラズベリー (生) |
- Vegetables and herbs with scarce flavonoids
The following vegetables and herbs have flavonoid contents less than 5 mg/100 g: beets, kidney beans, snap beans, cabbage, carrot, cauliflower, cucumber, endive, gourd, leek, lettuce, green peas, sweet pepper, potato, radish, tomato, oregano, perrilla, rosemary
Design of Flavonoid ID numbers
12-DIGIT
| F | L | x | x | y | y | z | z | w | c | c | c |
- x ... backbone structure (母核構造)
FL1 aurone and chalcone; FL2 flavanone; FL3 flavone; FL4 Dihydroflavonol; FL5 Flavonol; FL6 Flavan; FL7 Anthocyanin; FLI Isoflavonoid; FLN Neoflavonoid
- y ... hydroxylation pattern in A and B ring (水酸基パターン)
Click above categories to see details. General description is here.
- z ... glycosylation pattern (糖修飾パターン)
Click above categories to see details. General description is here.
- w ... halogenation etc. (ハロゲン等)
Currently unused.
- c ... serial number (通し番号)
For Users of Flavonoid Viewer
The flavonoid IDs used in this site is the same as those in Flavonoid Viewer in metabolome.jp except for the following FL7 category.
| Anthocyanin glycosylated with other than glucose and galactose | ||
|---|---|---|
| Flavonoid Viewer FL7A..GS |
→ | This site FL7A..GO |
Subcategories
This category has the following 10 subcategories, out of 10 total.